
The story of 1,2-Bis(Trimethoxysilyl)Ethane tracks alongside the rise of organosilicon chemistry in the late 20th century. Chemists working on silica-based materials realized that bridging organic molecules could link silane structures, making more durable and versatile materials. During the 1970s and 80s, researchers explored a variety of organofunctional silanes to expand the properties of composites and coatings. As industries demanded improved adhesives and sealants, the need for bifunctional silanes grew. This demand paved the way for regular use of 1,2-Bis(Trimethoxysilyl)Ethane. Companies and universities developed methods to control the reactivity and purity of this compound, so new applications kept emerging in both labs and factories. To this day, improved manufacturing methods keep rolling out, but the roots stretch back to that initial boom in curiosity and necessity.
At its core, 1,2-Bis(Trimethoxysilyl)Ethane is designed to build strong, flexible chemical bridges between organic molecules and inorganic surfaces. Its effectiveness depends on the combination of an ethylene 'bridge' and two silane ends. It works as a coupling agent, binding resins to glass, minerals, or metal oxides. Manufacturers rely on this compound in high-performance adhesives, coatings, and engineered composites. You'll find it in sealing products where moisture resistance counts, electronic encapsulants, and demanding construction materials. It remains valued by specialists who work with silicone rubber, glass fibers, and filled plastics, since the chemical bond it forms can improve the toughness and aging characteristics of the final material. In my experience, the reason people keep returning to this particular silane is simple—its unique balance of flexibility and bond strength suits the job in ways other additives can't match.
This compound appears as a colorless to pale yellow liquid, with a characteristic odor that hints at its methoxysilane content. It carries a molecular formula of C8H22O6Si2 and a molecular weight around 270.43 g/mol. The liquid state comes from the presence of three methoxy groups on each silicon atom, which prevents premature polymerization. It boils at close to 290°C and shows limited solubility in water, but dissolves well in many organic solvents like toluene or alcohols. The key to its utility lies in rapid hydrolysis once exposed to water, forming silanols that quickly condense into siloxane bonds. The process releases methanol, so the application environment needs careful control. Despite its reactivity, the compound remains stable in sealed containers at room temperature and away from moisture.
Manufacturers provide 1,2-Bis(Trimethoxysilyl)Ethane with purity levels typically above 97%. The liquid comes in containers resistant to corrosion, often labeled according to regulations set by OSHA and the Globally Harmonized System. You’ll see hazard pictograms, recommended storage temperatures, and warnings about flammability and irritancy. Each shipment includes a Certificate of Analysis outlining refractive index, purity by GC, and content of free methanol. For large-scale users, drum and tote sizes are available, always with attention to secondary labeling for workplace safety. Companies handling this compound blend regulatory compliance and practical handling advice, making sure anyone in contact with the product knows the risk factors.
Producers synthesize 1,2-Bis(Trimethoxysilyl)Ethane by reacting ethylene dichloride with trimethoxysilane in the presence of a catalyst. The technique often employs Lewis acids to encourage coupling while minimizing side reactions. Purification usually involves distillation under reduced pressure, taking advantage of the compound's thermal stability. Over time, process control has tightened to cut impurities that could impact performance, especially in sensitive electronic or medical uses. This focus on preparation yields a product that helps manufacturers meet exacting standards for their advanced materials.
The hydrolytic instability of the methoxy groups defines much of this compound’s chemistry. In the presence of water, each group hydrolyzes to form silanol, which can then condense with other silanols or with surface hydroxyls on glass or metal. The bridging ethane unit lets the molecule span gaps between surfaces, forming tough, flexible bonds. Chemists have also explored partial substitutions, where only some of the methoxy groups get hydrolyzed for controlled cross-linking. With the right catalysts, the compound can be integrated into sol-gel processes to give hybrid organic-inorganic frameworks. Some labs have even attached functional groups to the ethane bridge, tuning adhesion or reactivity.
1,2-Bis(Trimethoxysilyl)Ethane sometimes appears under trade names or as BTME. You may also see it referred to as Ethylene-1,2-bis(trimethoxysilane), or by the abbreviation TES. CAS numbers provide an unambiguous identifier, and catalogues from specialists like Gelest or Evonik list it under both systematic and shorthand names. Users familiar with silane chemistry will recognize related molecules, but the ethane bridge puts this one in a special category for flexible, dual-anchoring applications.
Work with 1,2-Bis(Trimethoxysilyl)Ethane requires personal protective gear and exhaust ventilation. Methanol release during hydrolysis brings both toxicity and flammability concerns. The liquid acts as an irritant to skin and eyes, so direct contact needs to be avoided. Facilities must follow spill containment procedures, with materials for absorbing both the compound and released methanol. Fire control relies on foam or dry chemicals. Waste disposal involves both local regulation and knowledge of environmental impact. Having seen best practices up close, effective training and good labeling make a huge difference in reducing workplace incidents.
Industries use 1,2-Bis(Trimethoxysilyl)Ethane in durable coatings for aerospace parts, construction sealants that withstand harsh weather, and encapsulants protecting microelectronic devices. The coupling agent helps glass fibers bond with resins, creating composites for automotive and marine markets. Manufacturers of adhesives appreciate the performance in formulations for bonding metals, plastics, and ceramics. In foundries, the compound modifies sand for improved casting surfaces. Tire and rubber industries also use it to enhance filler compatibility, leading to stronger, more reliable products. Researchers have identified new roles in the preparation of mesoporous materials and hybrid polymers, which feed into catalysts and membranes used in energy and purification technologies.
Ongoing research explores how 1,2-Bis(Trimethoxysilyl)Ethane can add value through customized surface treatments. Scientists look at the structure formed at the interface between organic polymers and inorganic fillers, using advanced microscopy and spectroscopy. In my own lab work, tweaking reaction times and temperatures improved the durability of resin-glass composites. Collaborative studies across universities and industry have probed its role in sol-gel processing, impacting fields as diverse as optics, separation technologies, and biomedical devices. Recent work also looks at alternatives to methoxy groups for even safer, more stable products.
Studies confirm that ingestion or inhalation of methanol, a hydrolysis byproduct, leads to organ damage and acute toxicity. Animal studies with the parent compound highlight the importance of limiting exposure, as high concentrations can cause respiratory and skin effects. Occupational health guidelines from bodies like NIOSH and OSHA offer strict exposure limits, emphasizing engineering controls and regular monitoring. Long-term studies continue, though short-term toxicity is better understood than chronic risks. Improved detection methods now support earlier identification of spills and leaks, lowering health risks for workers.
As composite materials gain ground in everything from lightweight vehicles to green energy, the need for strong, adaptable coupling agents keeps growing. 1,2-Bis(Trimethoxysilyl)Ethane will stay relevant by meeting the demanding standards for durability and environmental stability. New generations of the product may feature reduced toxicity and faster curing. Green chemistry pushes research teams to develop derivatives based on renewable methoxy sources or to capture and recycle byproducts. Economic pressure always encourages makers to improve yields and drive down costs, but quality remains a non-negotiable. New applications in nanotechnology, flexible electronics, and medical implants may reveal more about how this well-established molecule still has room to grow.
1,2-Bis(Trimethoxysilyl)ethane goes by a forgettable name in most homes but shows up in places a lot closer than you might think. I keep running into it when helping friends fix up tile floors or when builders want to make concrete last longer. This stuff holds a double role in construction, chemistry, and coatings that never gets much attention. You can spot its impact each time a kitchen backsplash stays put or a sidewalk survives another rough winter.
Anybody working with glass-reinforced plastics, or companies making adhesives that live up to their promises, runs across silane coupling agents. This chemical stands out with two different groups in its molecule: one end loves organic materials, one bonds to minerals. That split personality helps it act as a bridge. Add it to things like resin composites or specialty paints, and you suddenly have far less peeling and cracking, because it grippingly ties together materials that would otherwise break apart over time.
It helps concrete resist water, chemicals, and weather way longer. My own driveway has a few patches that were coated with additives like this years ago. The treated parts brush off winter salt and road grime like nothing, while the untreated ones crumble. In commercial construction, crews blend it in for final coating or surface protection. There’s plenty of data showing buildings with silane-treated surfaces shed rainwater and pollutants much faster, slowing down the usual decay caused by the elements.
Beyond the world of construction and home improvement, this silane compound helps with microelectronics and medical technologies. In labs, researchers coat glass or silicon surfaces with it so sensors and test strips respond faster or detect smaller things. These insights came up during a project I did measuring tiny electrical signals, where the chemical let us prepare glass so bonds stayed stable during high-heat cycles. Turns out, companies making circuit boards or compact sensors rely on this trick to save production time and reduce expensive waste from defects.
Every chemical with this much use comes with a story about care and safety. While working at a coatings manufacturer, I learned that even trace air moisture makes some silane agents release methanol. That’s not a small worry. It means any mixing, spraying, or disposal at a plant must stick to strong ventilation and spill protocols, or risk hazards for workers. Factories in the US and Europe track air quality and require gloves and goggles for anyone near the stuff on the shop floor. Talking to a safety supervisor, I saw the paperwork and monitoring routines—nothing gets left to chance.
Companies keep improving silane formulas to cut down on fumes or find less hazardous by-products. Green chemistry groups want these treatments to last even longer per application, so less gets washed away into water or air. Many new research leads chase the same promise: stronger bonds in more eco-friendly packages. With construction growth worldwide, especially in areas facing tougher climates, the pressure rises to develop safer and smarter coatings. That outlook keeps me watching, knowing that small improvements in chemical design shape how well our roads, bridges, and devices handle daily punishment.
1,2-Bis(Trimethoxysilyl)ethane carries the chemical formula C8H22O6Si2. Breaking it down helps to understand what it brings to the table: each molecule offers two silicon atoms, each surrounded by methoxy groups (three to each silicon). An ethane backbone links both silicons. For anyone spending time in a chemistry or advanced materials lab, these details quickly become more than academic. This molecule brings the building blocks necessary to form flexible, resistant, and durable siloxane networks. Businesses in coatings, adhesives, advanced composites, and sol-gel processing often rely on these very kinds of silanes for the physical characteristics customers expect—moisture resistance, longevity, and strong bonding to both inorganic and organic surfaces.
Knowledge about molecular structure is not just “classroom chemistry.” For those in the field, it helps to tweak product formulation, reduce product failures, and even predict regulatory hurdles. The three methoxy groups around each silicon atom in 1,2-Bis(Trimethoxysilyl)ethane react with water. This process, called hydrolysis, produces alcohol and silanol groups, eventually forming robust silicon-oxygen bonds. Users can create water-repellent barriers, drive adhesion between mineral surfaces, or finish high-performance coatings designed for long-term weather exposure. It is hard to ignore just how much reliability in modern building materials leans on these chemical details.
For the past decade, interest in sustainable construction and electronics has spiked. Today, engineers want alternatives to older, less durable bonding agents and coatings. 1,2-Bis(Trimethoxysilyl)ethane supports those needs. Chemical structure here supports crosslinking efficiency, which means products last longer and perform better with less environmental cost. Without the silane linkers found in this class of molecules, many “green” construction strategies would stall at the surface—literally. In my work with specialty glass coatings, using the right silane, such as this one, often spells the difference between films that delaminate within months and coatings that handle years of UV stress and scrubbing.
Manufacturing safe, responsible products requires respect for chemical details, down to every atom. Regulations worldwide now lean heavily toward transparency. Knowing whether a compound contains methoxy or ethoxy groups, for example, can change not just shelf life but health and handling guidelines. Many in the field recall the switch from poorly understood silanes to materials that passed standardized tests for emissions and workplace safety. Greater clarity about chemicals like 1,2-Bis(Trimethoxysilyl)ethane leads to fewer workplace injuries, fewer environmental releases, and fewer headaches when approvals are needed.
If chemists and engineers invest in up-to-date databases, better MSDS reporting, and clear labeling, mistakes drop. The best-run teams make sure scientists, packagers, and safety personnel all understand the formula C8H22O6Si2. When teams know what’s in the drum, waste falls, surplus spending drops, and fewer accidents send people home early. Science supports business, and details like these—chemical formula, structure, reactivity—allow responsible innovation.
1,2-Bis(Trimethoxysilyl)ethane isn’t your average shelf chemical. Its use spans rubber, coatings, adhesives, electronics—fields where a single misstep in storage can mean wasted product, safety headaches, or expensive cleanup. Silyl compounds like this draw moisture from the air, setting off reactions and producing methanol as a byproduct. That methanol needs respect; it’s flammable and toxic. The first thing that comes to mind for anyone who’s spent time with chemicals like these: humidity is the enemy.
In my years around research labs and chemical supply rooms, I’ve seen well-labeled glass bottles sitting on benches, and I’ve seen substances like this go to waste simply because they spent too much time with the cap left loose. Sometimes, the cost comes down to cleaning out ruined stock or, worse, dealing with pressure that builds up in containers. Methanol buildup can put workers at risk; it’s not worth cutting corners. Facts show that improper storage triggers hydrolysis, leading to pressure, leaks, and waste.
At the end of the day, storing this kind of silane boils down to three essentials: containment, dryness, and temperature. Screw-top, airtight bottles—ideally amber glass—block moisture and light. Plastic can work, but not every plastic holds up against organosilanes for the long haul. Once I watched a bottle stored in the wrong plastic start to deform; not a pretty sight. Storage rooms or cabinets should keep temperatures steady, well away from direct sunlight and the heat cycling found near radiators or vents.
Anyone who thinks about bulk storage quickly realizes ventilation means more than just comfort. Methanol vapors accumulate in stuffy spaces, which signals trouble if a leak occurs. Splash protection helps, but so does keeping an eyewash station nearby. Segregating chemicals also plays a huge role. Silyl compounds shouldn’t sit next to acids, strong bases, or oxidizers. This goes back to the lessons learned after minor accidents: one batch of a spilled acid can ruin more than your shoes if it hits the wrong container.
It’s easy to overlook labeling—until someone grabs the wrong bottle during a rush job. All bottles should have the name, concentration, hazards, and storage date. Make sure the storage location has clear signage with emergency procedures laid out. I’ve seen the chaos that follows after a poorly labeled stockroom; it puts everyone’s health, and even company reputation, on the line.
Periodic inspections spot crusty caps or pressure buildup before problems escalate. Rotating stock makes sure older supplies get used up before degradation sets in. I always respected the labs that ran tight logs; expiration dates weren’t ignored, and surprise leaks never caused panic. Saving money goes hand-in-hand with ensuring safety, and detailed records can keep everyone honest.
No system stands a chance without people understanding the why behind the rules. Every new technician I mentored had to run through safe handling procedures, not just skim a sheet. Open conversations about near-misses help catch blind spots before the next shift rolls in. Sharing stories from the field builds a culture where everyone looks out for one another, and nobody forgets the risks lurking in those innocuous-looking bottles.
Storing 1,2-Bis(Trimethoxysilyl)ethane safely sends a message: attention to detail isn’t just bureaucracy—it keeps lives and livelihoods intact. Those of us who’ve seen accidents know proper storage is never wasted effort. That’s a lesson the industry can’t afford to relearn the hard way.
At first glance, 1,2-Bis(Trimethoxysilyl)Ethane sounds like the kind of chemical only a scientist could love. In reality, many people cross paths with it when working in construction, coatings, or adhesives. It plays a big part in producing some high-performance industrial materials—helping concrete stick together, protecting surfaces from wear, and even linking different chemicals together in new, useful ways.
Just because a compound helps build sturdy skyscrapers or slick waterproof coatings doesn't mean it's free of health risks. Ask any chemist or site manager about handling 1,2-Bis(Trimethoxysilyl)Ethane, and you’ll hear about gloves, goggles, and proper ventilation. Most safety data points out concerns with skin and eye contact. Direct splashes can irritate or burn, and breathing in large amounts of vapor may trigger symptoms like headaches or a sore throat.
OSHA and other occupational safety groups recommend treating it like any other industrial chemical: Keep your exposure low and avoid breathing dust or vapor. One strong argument for caution, in my view, comes from the way it breaks down. In humid air, this compound forms methanol—a well-known toxin. Methanol exposure through inhalation or skin absorption carries a real risk of neurological symptoms, vision problems, or worse if left unchecked.
Large spills rarely reach streams and fields, but a careless approach could lead to long-term damage. Its breakdown products can harm aquatic life if they find their way into water sources. Over time, methanol can disrupt fish and invertebrates, which shows that safe handling matters beyond the immediate workspace. Companies should keep spill kits nearby and make sure workers feel confident about containment practices.
I've seen plenty of facilities address these concerns with straightforward steps—using closed systems, updating safety training, and checking air quality regularly. Personal experience suggests that many workers trust simple common sense more than a thick safety manual. Basic moves like wearing chemical-resistant gloves, goggles, or face shields, and avoiding skin contact go a long way.
Safety teams can help by labeling storage drums clearly and reviewing procedures before each big delivery. One plant manager I know took this a step further by encouraging workers to talk openly if they saw unsafe storage or handling. Peer checks make it easier to catch small mistakes before they snowball.
Protecting your own health—along with everyone's around you—means understanding both the chemistry and the people who use it. If companies offer good ventilation, reliable personal protective equipment, and backup plans for spills, people notice. Familiar routines like washing up after a shift, skipping food in the work zone, and reporting accidents all add layers of safety.
It comes down to respect for the work and one another. Industry watchdogs and safety agencies list these protections for a reason. As long as real humans handle 1,2-Bis(Trimethoxysilyl)Ethane, we owe it to ourselves to take precautions seriously. That respect keeps small problems from growing into big ones.
Anyone who's stepped into a lab or worked with chemicals understands that one overlooked detail or shortcut can turn a routine day into a regretful one. 1,2-Bis(Trimethoxysilyl)Ethane doesn’t sound particularly dangerous—its name is more intimidating than the liquid itself—but as with many organosilane compounds, ignoring the hazards sets up trouble.
This silane compound gives off methanol during hydrolysis. Methanol carries real health risks. Breathing it in, getting it on your skin, or swallowing just a small amount can cause serious health issues. Folks who work around this chemical aren’t just handling another bottle—they’re up against fumes that don’t always announce themselves with a strong smell or immediate irritation. That silent risk always sits in the background.
The liquid also reacts to moisture in the air. Left open, the container can quickly build pressure or lead to clogged lines and sticky, unwanted residues. I once watched a lid pop off and spray the bench; that stuff sticks and stays sticky for days. Emergency showers aren’t just for show in these workspaces.
Gloves and eye protection make a difference—nitrile gloves block splashes, and goggles keep eyes out of danger. Lab coats do more than complete the uniform. They protect from accidental drops and splashes—real risks anyone working with organosilanes faces. People often get careless and skip a step, only to regret it.
Solvent-resistant gloves matter. It surprises some folks to find out that the thinner latex ones break down fast with silane exposure. Thick nitrile or even neoprene offers a decent buffer between skin and chemical. Wiping up spills with paper towels doesn’t cut it; controlled, absorbent pads do a better job and help avoid secondary exposure.
Fume hoods aren’t just expensive furniture. Every time I’ve handled this compound, the process took place under well-maintained ventilation. The reason is clear: methanol vapor seeps into the lungs easily and acts quietly. Fans, extraction systems, and open windows reduce the odds of hidden danger, but a certified hood sets the bar higher, catching vapors before they can spread.
Flammable storage cabinets serve a real purpose. Not every lab has a separate explosion-proof fridge, but good practice means segregating organosilanes from oxidizers, acids, and water-reactive materials. Labels must stay easy to read—faded writing has led more than one technician to grab the wrong compound in a rush. Fortunately, dated and labeled bottles help avoid mix-ups.
Accidents don’t just risk personal health—they can force costly shutdowns, ruin research, or even attract scrutiny from safety authorities. Small spills grow into bigger issues when not contained immediately, and ignoring little rules about ventilation or gloves leads to long-term health risks, not just short-term injury.
Training makes all the difference. I have seen teams skip refreshers because they feel confident, but context shifts or new faces join the crew, and basic errors start slipping in. Clear procedures, fit-for-purpose gear, and attention to detail turn a hazardous chemical into a useful tool rather than a source of fear. Keeping safety data sheets handy, running drills, and double-checking storage arrangements—it’s the repetitive, almost boring details that protect people every single day.
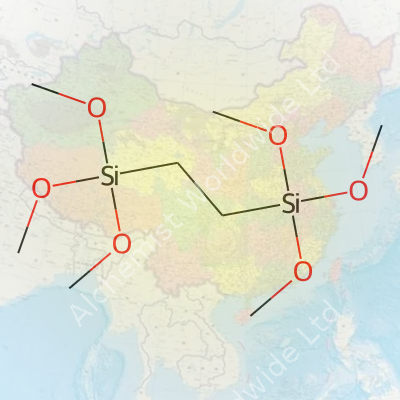
| Names | |
| Preferred IUPAC name | Bis(trimethoxy(silanyl))ethane |
| Other names |
BTMSE bis(trimethoxysilyl)ethane ethylene-1,2-bis(trimethoxysilane) 1,2-bis(trimethoxysilyl)ethane trimethoxy[2-(trimethoxysilyl)ethyl]silane |
| Pronunciation | /ˈwʌnˌtuː bɪsˌtraɪˌmɛθɒksiˈsaɪlɪl ˈiːθeɪn/ |
| Identifiers | |
| CAS Number | [18406-41-2] |
| Beilstein Reference | 1465068 |
| ChEBI | CHEBI:87156 |
| ChEMBL | CHEMBL3184707 |
| ChemSpider | 21542189 |
| DrugBank | DB11204 |
| ECHA InfoCard | ECHA InfoCard: 100.107.504 |
| EC Number | 216-925-4 |
| Gmelin Reference | 92253 |
| KEGG | C19368 |
| MeSH | D017131 |
| PubChem CID | 98617 |
| RTECS number | KV4900000 |
| UNII | QG8Y47DA5M |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | 'DTXSID3020685' |
| Properties | |
| Chemical formula | C8H22O6Si2 |
| Molar mass | 238.38 g/mol |
| Appearance | Colorless to pale yellow transparent liquid |
| Odor | Odorless |
| Density | 1.04 g/mL at 25 °C (lit.) |
| Solubility in water | slightly soluble |
| log P | 0.8 |
| Vapor pressure | <0.01 hPa (20 °C) |
| Magnetic susceptibility (χ) | -7.55E-6 cm³/mol |
| Refractive index (nD) | 1.4200 |
| Viscosity | 5 cP (25 °C) |
| Dipole moment | 2.45 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 395.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1464.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1781 kJ/mol |
| Pharmacology | |
| ATC code | No ATC code. |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS05 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P305+P351+P338, P321, P332+P313, P362+P364, P337+P313, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 104 °C |
| Lethal dose or concentration | Acute Toxicity (Oral - Rat) LD50: > 2000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): > 5000 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 2–8°C |
| Related compounds | |
| Related compounds |
Trimethoxysilane Tetramethoxysilane 1,2-Bis(triethoxysilyl)ethane Vinyltrimethoxysilane Methyltrimethoxysilane Phenyltrimethoxysilane |