
Chemistry rarely stands still, and the journey of 2-aminoethylamino methyl triethoxysilane reflects this restless movement. During the 1970s and 1980s, as industries grew hungry for materials that bond dissimilar surfaces, silane coupling agents drew a lot of attention. Certain companies chasing better adhesives and coatings found that introducing specific organofunctional groups made significant improvements. The amine-functional silanes, such as this one, appeared as a logical answer, building on both the practicality of siloxane chemistry and the commercial need for more adaptable agents. Sourcing, scaling, and purifying such a molecule became a focus, with teams working late in labs, balancing hydrolysis resistance and reactivity. Anyone who’s mixed a trialkoxysilane with water and watched a rapid gelation understands just how finicky these materials can behave in real world conditions. Each step in commercializing these compounds grew out of this back-and-forth—basic science feeding application and vice versa.
2-Aminoethylamino methyl triethoxysilane lands somewhere between a mere laboratory curiosity and a must-have industrial additive. In simple terms, its core structure displays both a reactive amine and a triethoxysilane group. That unique design means it not only takes part in organic reactions but also actively couples with inorganic surfaces like glass and metals. Most suppliers offer a clear, colorless to pale yellow liquid. Anyone who’s worked with it knows it has an unmistakable ammonia-like odor, so it sticks out compared to most bland silanes. Its ability to promote adhesion, modify surfaces, and act as a primer makes it more than a niche product; with the right setup, it can improve both manufacturing yield and final product performance for countless formulations.
This silane has the feel of a solvent—light, free-flowing, with a boiling point typically near 263°C. Handling it in a lab without gloves often leads to a sharp, tingling sensation on the skin, which speaks directly to its high reactivity. The amine groups draw in moisture from the air, a fact that keeps warehouse managers vigilant about drum lids and air-tight packaging. On paper, you’ll see molecular formulas like C8H22N2O3Si, with a molecular weight around 222 grams per mole. Soluble in standard polar solvents and prone to hydrolytic breakdown in water, its triethoxysilyl group can condense to form robust siloxane bonds over time, which adds another layer of utility in both cross-linking and surface grafting applications.
Manufacturers offer up data sheets showing purity commonly above 97%, occasional mention of heavy metals under strict thresholds, water content below 0.5%, and color measured on the Gardner scale. Labels emphasize the need for moisture control, warnings about skin and eye irritation, and the various synonyms—like N-(2-aminoethyl)-3-aminopropyl triethoxysilane or simply AEAMTES. Bags or drums usually arrive packed with a batch number and recommended storage temperature, since a warm warehouse or a leaky seal turns this asset into a liability quickly. In the field, keeping those labels legible and double-checking certificates of analysis becomes routine, especially where regulatory audits get involved.
I remember a time prepping for a batch synthesis, carefully measuring 3-chloropropyltriethoxysilane and reacting it with ethylenediamine under controlled temperatures. The exothermic nature of the reaction meant coolant lines never sat idle. Post-reaction, purification required vacuum distillation to clear out the unreacted starting material. It’s a process that rewards patience and a steady hand, especially since even a small contamination affects downstream properties. Industrial-scale synthesis certainly reduces a portion of the manual labor, but core steps remain: adding the amine to the silane backbone in a controlled environment, purifying, then bottling under nitrogen or another inert gas. Knowing how sensitive this compound is to atmospheric moisture means techs often check and recheck every seal and joint.
On a molecular level, both the amine and silane groups are ready for action. Chemists often exploit the amines to introduce dyes, protective groups, or reactive cross-linkers, while the triethoxysilyl tail hydrolyzes in the presence of trace water, anchoring firmly to silicon, glass, or metal oxides. Surface modifications for chromatography columns or biomedical implants almost always hinge on this basic chemistry. In my own projects, grafting this silane onto quartz yielded tailored surfaces that outperformed older, non-functionalized alternatives. Over time, researchers rolled out numerous derivatives, taking N-alkyl substitutions and even attaching fluorescent tags for sensor applications. Breaking the molecule up or linking it into siloxane networks extends its reach, making it a backbone for diverse adhesives, sealants, or even electronic encapsulants.
Walk into a chemical storeroom and you might spot several names on the warehouse racks—N-(2-aminoethyl)-3-aminopropyltriethoxysilane, AEAMTES, and silane coupling agent A-1120 being just a few. Commercial catalogs from companies like Evonik, Momentive, or Gelest list dozens of close analogues, each with slight tweaks or branding differences. This proliferation of synonyms often stumps newcomers, yet it points to the broad utility and demand across glovebox labs, pilot plants, and mass production lines. Making note of CAS numbers alongside trade names helps in avoiding costly mix-ups.
There’s no escaping the safety aspect of working with amine-functional silanes. Direct contact brings burning sensations, and inhaling vapors leaves the airways irritated, sometimes long after exposure. Modern operations, especially those ISO-certified, enforce sealed handling systems, chemical splash goggles, nitrile gloves, and plenty of fresh air through hoods or local exhaust systems. Spills rarely linger, as even residues can stain or etch surfaces. Waste disposal usually needs coordination with specialized chemical services—throwing this stuff into ordinary drains counts as poor practice and creates knock-on problems downstream. Documentation follows standard GHS hazard formats, with stringent training for any line or maintenance workers dealing with the material. Regular reviews and near-miss records keep operations sharp, reducing both personal risk and cost from downtime or lost product.
Demand for this silane crosses boundaries—electronics, coatings, composites, adhesives, and even emerging fields like biointerface engineering. PCB manufacturers use it to prime glass-fiber surfaces, leading to stronger, more durable boards. Paint formulators have found that including this silane can mean the difference between a flaking finish and years of service. Composite material designers see improved matrix-fiber bonding and toughness by applying this agent. From personal experience, using this silane as a surface primer in high-reliability aerospace assemblies made a measurable difference: bond failures dropped, water ingress issues nearly vanished, which cut down on both warranty returns and operational headaches. Biomedical researchers push farther, testing it for functionalizing implant surfaces or biosensor electrodes. New ideas launch almost weekly as teams discover fresh combinations with this versatile molecule.
For every published paper on surface science with this silane, there’s probably an internal project in some R&D division aiming at either incremental or disruptive improvements. Scientists often blend it with other silanes or silylated polymers, tuning surface energy or bonding profiles for new substrates. Academic labs, especially those focusing on sustainable or “green” chemistry, tweak synthesis routes with recyclable catalysts or less hazardous solvents. Instrumented labs measure everything from surface roughness to spectroscopic confirmation of successful grafting. Tech transfer teams typically focus on translating bench-scale protocols to pilot production, negotiating yield, purity, and regulatory demands. Innovations explode when collaboration happens between sectors—military technology finds ways to harden materials, while medtech groups use similar ideas for anti-fouling or anti-bacterial surfaces.
Safe use doesn’t happen by accident, and toxicity studies remain crucial. Animal testing shows potential skin and eye irritancy, but major regulatory agencies have yet to flag significant long-term carcinogenic risks at usual exposure levels. Chronic inhalation sits as a bigger worry, especially for those in downstream bulk processing. Years spent mentoring junior lab techs reinforced a key habit: treat all amine silanes respectfully, minimize direct contact, and always document exposure. Disposal practices get reviewed regularly, since breakdown byproducts can show aquatic toxicity. Environmental monitoring now forms a routine part of operations at larger plants, with local communities pushing for even stricter limits on workplace exposure and effluent.
New frontiers keep opening for amine-functional silanes. The growing push toward smart materials, hybrid composites, and greener manufacturing methods gives this molecule a second wind. Companies pursuing improved tire reinforcement, next-generation solar panels, or responsive coatings push for higher performance at lower costs. Researchers in biointerface science push for cleaner, more effective means of surface modification, sometimes harnessing enzymatic or light-triggered reactions. All this innovation loops back to driving demand for robust, adaptable linking agents, and anyone following trade news sees new patents and application reports roll out every quarter. As regulatory landscapes evolve, the future likely brings not just tweaks to product safety or handling but new formulations that do more with less environmental impact. Whether in scalable manufacturing or frontier research, this silane sits at an exciting juncture—a simple molecule shaping complex possibilities.
2-Aminoethylamino methyl triethoxysilane doesn’t show up in everyday conversations, but anyone working in industrial coatings, adhesives, plastics, or even advanced electronics recognizes it fast. This is a silane coupling agent, which means it forms crucial bridges between chemicals that normally don’t stick together. Think of it as the glue that lets oil and water find a common language. Plenty of new materials—especially the tough composites used in cars, planes, and microchips—come down to secrets like this one.
On shop floors mixing epoxy, folks see how this silane agent works up close. Fiberglass parts, for example, often come out stronger because their glass fibers accept resin more willingly after a dose of 2-aminoethylamino methyl triethoxysilane. The chemical’s amine group bonds with plastics while the silane side grabs hold of glass or minerals. Performance stats like higher flexural strength and better resistance to heat track back to this hidden ingredient.
Paints and coatings don’t just need color and shine—they need to survive all kinds of abuse. Salt spray, sun, oil splashes—these hammer away at coatings on bridges, tanks, and wind turbine blades. A silane like this one becomes the link in the chain, tying pigments and resins to metal or concrete. Some years ago, I worked on maintenance coating guides for steel infrastructure and, over and over, I saw labs pick this molecule because it helped the coating last through cycles of wet and dry.
Advanced semiconductors and printed circuit boards have to balance signals, heat, and reliability all at once. Engineers often pretreat glass or ceramic surfaces with a solution containing 2-aminoethylamino methyl triethoxysilane. This helps later layers of polymers lock onto the substrate, cutting down on failures from humidity or cycling. Between tight deadlines and even tighter specs, crews rely on surface prep that works every time, and this agent provides that invisible assurance.
New uses pile up, but it makes sense to look at safety and environmental effects before mixing up a fresh batch. This silane, just like many amine-containing chemicals, can irritate the skin and eyes. Proper gloves and safety goggles remain non-negotiable. Lots of manufacturers have moved toward closed handling systems and better ventilation to match stricter occupational safety rules.
For disposal or releases, the question isn’t just about policy—it’s about keeping waterways and air clean for everyone. Research suggests that silanes like this break down relatively quickly, but incomplete reactions or bad disposal practices can leave traces where we don’t want them. Following clear protocols and using the right waste management strategies—incineration or chemical neutralization—matters more now than ever.
Demand keeps growing for composites and corrosion-resistant coatings. The industries adopting this silane tend to push old technologies aside for lighter, tougher, longer-lasting solutions. My own experience talking with engineers and chemists shows that, every year, the demands get more specific. Adjusting processes to minimize exposure and waste lines up with the global push for safer, greener chemistry. This is where trusted technical data, careful user education, and attention to best practices shouldn’t lag behind industrial progress.
Dealing with chemicals like 2-Aminoethylamino Methyl Triethoxysilane isn’t just a matter for lab specialists—many of us in coatings, adhesives, or advanced materials find our hands in the mix more often than we'd like. Ignoring proper handling turns a useful tool into a legitimate hazard. Eyes, skin, and lungs are all targets for this compound if we’re not careful. Learning real-world ways to keep those risks down saves jobs and keeps folks out of the ER.
It doesn’t take a major spill to end up regretful. Vapors or droplets can cause quick irritation, and in bad cases, burns or breathing trouble. In my experience, basic gloves aren’t enough. The material chews through thin latex. Nitrile holds up better, but it shouldn’t be the last line of defense.
Common sense sometimes fails in busy workshops. I’ve seen people pour directly without goggles, banking on luck. One slip in a humid room, splash bounces up—I’ve never forgotten how fast chemical burns happen around the eyes.
Wearing splash goggles and face shields needs to become as automatic as tying a boot before leaving the house. In my shop, thick nitrile gloves get swapped the minute they show any pitting or weakness. Disposable lab coats or Tyvek suits cut down on skin exposure, and I always keep a shower and eyewash station in clear sight and fully functional.
Ventilation counts for just as much as the right gear. Open bays, decent fans, or purpose-built fume hoods push vapors away before they build up in your lungs. Time and time again, I’ve watched rooms get stuffy from neglecting ventilation, and people start to cough, headaches follow, sometimes nausea. OSHA recommends keeping airborne concentrations far below established exposure limits, and I check meters before crack a fresh drum.
It’s tempting to stash leftover jugs in the nearest closet, but these silane compounds play rough with water and release hazardous fumes if they react with moisture. Sealed, clearly labeled containers—preferably glass-lined with good gaskets—should go in a dry, cool spot. I’ve had containers bulge or leak after sitting in humid corners.
Separate storage pays off, too. Keeping acids and bases in different cabinets shields you from nasty cross-reactions. It’s not just about big accidents—even minor mixing can create pressure or release gases that damage property (or people).
Once a container’s done, it’s not just empty plastic. Residue still acts as a chemical hazard. I always rinse thoroughly with solvents recommended on the Safety Data Sheet (SDS) and keep rinsate for hazardous waste pickup. Pouring leftovers down the drain risks both personal injury and environmental fines. At our facility, licensed industrial disposal services pick up both waste and used PPE.
Mistakes happen even with experienced crews, especially if everyone isn’t on the same page. I take hands-on training seriously—regular reviews of the latest chemical safety info and emergency response practice. For me, trust grows every time someone asks questions, runs a drill, or double-checks a label. Keeping up with evolving standards, from OSHA to the EPA’s Hazardous Waste requirements, gives both newcomers and veterans peace of mind.
Staying safe around 2-Aminoethylamino Methyl Triethoxysilane relies more on habits and honesty than luck. We owe it to ourselves, our coworkers, and anybody downstream to treat every container with care and respect.
Working with chemicals like 2-Aminoethylamino Methyl Triethoxysilane isn’t just about what happens in the lab—it’s about the storage space behind closed doors. Putting safety and quality first matters, especially with substances that bring both promise and risk. My own experience tells me one overlooked drum or a shortcut on shelf labeling can set a safety team back for weeks or worse. With this compound, the details can’t be skipped.
Let’s talk about the stuff people gloss over. 2-Aminoethylamino Methyl Triethoxysilane can react with moisture, release ethanol fumes, and eat away at metals or coatings. Those aren’t abstract chemistry-class warnings; they’re real possibilities. Handle an old, leaky bottle, and you’ll catch a whiff of alcohol that lingers in your nostrils—and that’s your reminder to avoid sloppy handling. Fire hazards? Check. Corrosive skin burns? They happen more than you’d think. Anyone who’s uncapped a slightly sticky bottle in a hot room will know what I mean.
No fancy vocabulary here: keep this stuff in a cool and dry place—room temperature works, but if your facility swings hot, upgrade your storage. I’ve seen containers warp and seals peel away after a single hot summer, so don’t gamble with a sunlit shelf. Humidity invites chaos. Moisture in the air can sneak inside even a supposedly sealed drum and start the reaction you’re trying to avoid. Desiccant packs or a dry cabinet keep things safer for the long haul.
Use original, factory-sealed containers until the last possible moment. If you transfer to smaller bottles, make sure they’re clean, dry, tightly sealed, and made of materials that stand up to silanes. Glass works, but I’ve also seen high-density polyethylene hold up just fine. Don’t tuck these bottles next to acids, bases, or water sources—mixing accidents and vapor cross-talk in storage racks are real problems, not just hypothetical worries.
It’s tempting to scribble a quick label or reuse a marker-stained sticker, especially on busy days. Resist the shortcut. Fresh labels with clear hazard warnings and expiration dates keep everyone safer—especially folks who didn’t stock the shelves or switch the cap last week. I recommend posting a reference chart nearby so anyone pulling a bottle knows what gloves and goggles they need. If your team takes a few extra seconds to check the chart or label, they’ll thank you in the long run.
Good airflow helps. Chemical fumes hang in the air more than people expect, especially if caps aren’t tightened all the way. Open storage in a ventilated flammables cabinet or a dedicated chemical fume hood makes a big difference. Spills still happen; keep absorbent pads within reach and train the team to not just mop up, but notify supervisors and document the event. One missed spill can turn a storage area into a hazard zone fast.
Nobody likes paperwork, but keeping storage logs up-to-date isn’t negotiable. If you know how many liters you started with, how much gets used each week, and who accessed the chemical, then you’re less likely to run out, lose track, or let an expired batch slip through. In my own labs, this diligence saved us a lot of wasted time and money—and kept compliance officers happy during surprise visits.
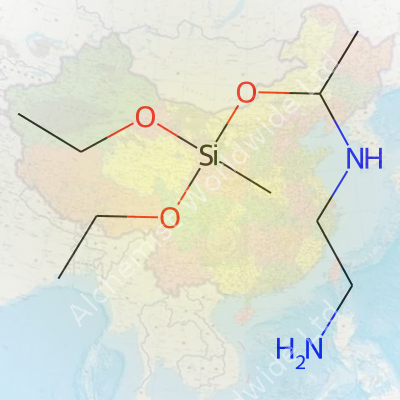
A molecule’s structure steers both its behavior and its uses. For 2-Aminoethylamino Methyl Triethoxysilane, the chemical formula is C8H22N2O3Si. Picture it as a silane backbone—the core silicon atom—hinged to three ethoxy groups (–OCH2CH3). A methyl bridge attaches to an aminoethylamino side chain. This side chain includes two nitrogen atoms separated by an ethylene group, joined through single bonds.
The condensed formula tells its own story: NH2CH2CH2NHCH2Si(OC2H5)3. Each part clues you into how the molecule behaves. The triethoxysilane part makes it eager to bond with inorganic surfaces like glass, metals, and minerals, while the amine groups connect well with organic materials, including resins and plastics.
After years spent in labs and manufacturing facilities, I’ve seen firsthand how this molecule gets pressed into service, especially during the push toward more durable, high-performance materials. Used as a silane coupling agent, it acts like a bridge—joining organic polymers to inorganic fillers or glass fibers. Without this bridge, paints flake, adhesives fail, and composite materials lose their strength. Cars, ships, construction panels, even some medical devices rely on chemical bonds that only emerge with the help of coupling agents like this one.
The amino groups on 2-Aminoethylamino Methyl Triethoxysilane don’t just add function—their presence changes the way it reacts during formulation. They anchor tightly to resins, making finishes more resistant to moisture, physical stress, and age. Technical literature supports this advantage: studies in the Journal of Adhesion Science show a direct link between amino-functional silanes and improved mechanical properties in filled composites and coatings.
Factories that use this chemical need strict ventilation, gloves, and eye protection, as it can irritate the skin, eyes, and breathing passages. I remember a facility audit where an engineer showed us the result of skipping basic controls—just a few minutes’ exposure led to redness and coughing. The European Chemicals Agency and national agencies require labelling and safe storage to protect workers and the environment.
Waste management gets equal attention. By following the REACH and OSHA guidelines, producers cut down on emissions and runoff, keeping the water table cleaner and air fresher around industrial sites.
With demand for better composites and coatings on the rise, supply chains can get tight. One solution comes from advancing synthesis methods—continuous flow reactors trim waste, offer better purity, and increase worker safety. Investment in green chemistry holds promise, as researchers seek ways to produce functional silanes with fewer toxic by-products.
Some industries chase alternatives based on biopolymers, but so far, few match silanes for reliability, especially in heavy-duty environments. Until science delivers new answers, companies must embrace stewardship—sharing best practices, testing regularly, and supporting worker education as ways to ensure safe, sustainable growth.
In research and production, facts about chemical safety, performance, and environmental impact must come from data, peer-reviewed publications, and direct experience. As customers and regulators tighten standards, only those who combine applied know-how with honest communication earn trust—and keep everyone safer.
I’ve seen a lot of lab shelves, and a bottle labeled 2-Aminoethylamino Methyl Triethoxysilane never stands alone for long. Folks who treat glass, metals, or even ceramics know this compound can be a go-to for building a bridge between an inorganic base and whatever organic layer ends up on top. Surface science isn’t just theory—it’s about getting paint to stick, or making a medical device work better. Getting this silane to play nice with other chemicals matters for every coating, adhesive, and lab experiment that turns into a real product.
This silane offers both amino and ethoxysilane groups. The amino part reaches out to epoxies, polyurethanes, and other organics. The silane moiety reacts well with glass, silica, aluminum, or stainless steel after some activation. Every chemist who’s tried has mixed it into epoxy and watched the mechanical strength shoot up. The trick is in the hydrolysis—the ethoxy group needs water to turn into a silanol. If there’s not enough water or the pH swings, expect patchy results.
Surface prep is everything. Dust, oils, or an oxide layer that's too thick can all wreck the outcome. I’ve messed this up more than once—just one careless swipe with a rag instead of a solvent wipe and the bond goes weak. Dry, clean, and slightly rough surfaces set the stage for a strong chemical handshake.
This silane bonds beautifully with glass, metal oxides, and some ceramics. Polyethylene or Teflon won’t care about all the chemistry—those surfaces shrug off most attempts unless they’ve been specially treated. Solvents make a difference; alcohols work smoothly, but strong acids or bases at high concentrations break it down or cause polymerization before it hits the surface. Mixing with other silanes can get tricky—some compete for the same surface spots. If you want a blend, test different ratios. Some formulations end up clouding or gelling faster than expected.
Coating over it? Epoxies, polyurethanes, and acrylates love the amine group. I once saw a composite delaminate after using a fast-curing epoxy with too little working time—the silane didn’t get a chance to set up. Reaction times, moisture levels, and even curing temperatures all play a role. Only testing in your own system gives the right answer for your mix of chemicals and surfaces.
Health and safety always enter the picture. Reliable sources like PubChem list moderate risks with direct exposure. Organic amines can irritate skin and eyes. Using gloves, goggles, and a proper fume hood keep things safe. Disposal rules vary, so checking local guidelines protects both people and the environment.
In my experience, actual bench tests are worth twice as much as spreadsheet predictions. Try small batches, tweak the surface prep, and keep notes. Avoid the temptation to skip straight to high-volume mixing. Even a slight change in one ingredient or the time spent between steps changes the final result. For folks blending new formulations or looking to upgrade industrial processes, starting with detailed compatibility testing and honest troubleshooting lays the foundation for reliable performance.

| Names | |
| Preferred IUPAC name | N-(2-aminoethyl)-N-(3-(triethoxysilyl)propyl)methanamine |
| Other names |
N-(2-Aminoethyl)-3-aminopropyltriethoxysilane 3-(2-Aminoethylamino)propyltrimethoxysilane Aminoethylaminomethyltriethoxysilane AEMTEOS Silane, N-[3-(triethoxysilyl)propyl]ethylenediamine |
| Pronunciation | /tuː əˈmiːnoʊˌɛθɪl əˈmiːnoʊ ˈmɛθəl traɪˌɛθɒksiˈsaɪleɪn/ |
| Identifiers | |
| CAS Number | 1760-24-3 |
| Beilstein Reference | 1703676 |
| ChEBI | CHEBI:64314 |
| ChEMBL | CHEMBL4160127 |
| ChemSpider | 26641718 |
| DrugBank | DB14185 |
| ECHA InfoCard | 03e6220d-4e18-4460-a97d-98e2ce93afd5 |
| EC Number | 220-941-2 |
| Gmelin Reference | 87536 |
| KEGG | C19215 |
| MeSH | 2-Aminoethylamino Methyl Triethoxysilane" does not have a specific MeSH (Medical Subject Headings) term assigned as of the latest update. |
| PubChem CID | 104966 |
| RTECS number | KY9177500 |
| UNII | WN4R2F7H9O |
| UN number | UN2735 |
| CompTox Dashboard (EPA) | DTXSID5054201 |
| Properties | |
| Chemical formula | C10H25N2O3Si |
| Molar mass | 234.38 g/mol |
| Appearance | Colorless to pale yellow transparent liquid |
| Odor | Ammonia-like |
| Density | 0.955 g/mL at 25 °C |
| Solubility in water | Miscible |
| log P | -1.2 |
| Vapor pressure | 0.48 hPa at 25 °C |
| Acidity (pKa) | 10.2 |
| Basicity (pKb) | 6.85 |
| Refractive index (nD) | 1.450 |
| Viscosity | 5-25 cP (25°C) |
| Dipole moment | 2.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 237.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H314 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P321, P330, P332+P313, P337+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 82 °C (180 °F) |
| Autoignition temperature | 270 °C |
| Lethal dose or concentration | LD50 (Oral, Rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 Oral Rat: 1390 mg/kg |
| NIOSH | B2-527 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Aminoethylamino Methyl Triethoxysilane: Not established |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
N-(2-Aminoethyl)-3-aminopropyltrimethoxysilane 3-Aminopropyltriethoxysilane 3-Glycidyloxypropyltrimethoxysilane N-(2-Aminoethyl)-3-aminopropyltrimethoxysilane hydrochloride Aminopropyltrimethoxysilane |