
Industry didn’t always have the sophisticated surface modifiers it relies on today. Back in the 1960s and 1970s, research groups proved that adding functional groups to organosilicon compounds could unlock new ways to engineer plastics and coatings. The family of methyl dichlorosilanes drew a lot of attention, since those products paired silane reactivity with organic side chains. Later, researchers added acetate-protected hydroxyls, opening up even more possibilities for targeted reactions. Chemists set off on a pursuit: deliver high-value silicone intermediates designed for ever-tightening safety and environmental rules. By the 1980s, trial batches showed that 3-acetoxypropyl methyl dichlorosilane could reliably link organic and inorganic phases. It didn’t take long before the compound made its way into commercial labs—often tucked away under custom manufacturer lots, waiting for a surge in demand from electronics and advanced materials research.
3-Acetoxypropyl methyl dichlorosilane presents itself as a versatile building block. It couples a hydrolyzable dichlorosilane group with an acetate-protected propyl tail, letting chemists toggle between rapid silylation reactions and later deprotection. Storied among silicon-based crosslinkers, its biggest claim to usefulness lies in that protected propyl chain. The presence of an acetate group keeps unwanted side reactions at bay, giving extra shelf-life and more predictable reactivity in batch processes. Manufacturers of specialty adhesives or coatings saw the advantage, especially in products where controlling interfacial chemistry can make or break the end-use properties.
Most labs who handle this compound know it appears as a clear to pale yellow liquid. Its boiling point falls in the 200–220°C range, though it starts to react with water long before reaching those temperatures. Its vapor can irritate eyes and the respiratory tract. In a sealed container, you might smell acidic fumes from hydrolysis, especially if moisture sneaks past the cap. As a methyl-substituted silane, it exhibits robust Si–Cl bonds—these cut through water or alcohols instantly. In my experience with silane chemistry, basic glassware prep falls short. You have to use dry nitrogen or argon and keep all reagents anhydrous. Otherwise, you walk into a mess of hydrochloric acid and sticky oligomers, and good luck clawing back purity or performance after that.
Suppliers usually offer this chemical in moisture-tight bottles, triple-sealed, and labelled by its CAS number and purity (usually above 97%). Key technical specs include weight per mole (chlorine atoms help tip it over 220 g/mol), refractive index (good for verifying batch purity in the lab), and residual acid content—an important check on unwanted hydrolysis. The labels list warnings for corrosivity and respiratory hazard, because you need to respect the fuming potential when working at scale. In practice, that means full-face shields, heavy gloves, and a backup neutralization bath standing by. The industry never wants another highly-publicized chemical exposure. Stringent documentation now traces this compound from manufacturer to end-user, backed up by SDS sheets and shipping manifests.
Industrial synthesis favors the direct hydrosilylation of vinyl acetate with methyl dichlorosilane. Platinum-based catalysts drive the addition across the double bond, inserting the acetate group at the tail of the propyl chain. This process requires meticulous control of temperature, gas feed, and catalyst site density to prevent side reactions. Any slip leaves you with unwanted ditertiary, over-reduced, or polysilylated by-products, which spell trouble for downstream uses. Outfitting a pilot line for this chemistry means investing in lined reactors, scrubbers for HCl off-gas, and rigorous analytics to monitor batch uniformity. My time in a process plant left me with a respect for the operators who keep water out of the loop and keep hydrochloric acid emissions within legal limits—no easy task as regulatory rules on air quality have only gotten tighter.
In actual use, the dichlorosilane moiety offers fast, high-yield coupling to surfaces containing hydroxyl groups, whether glass, metal oxides, or cellulose fibers. The acetate on the propyl chain can be cleaved in a mild base or upon heating, freeing a terminal alcohol which unlocks further functionalization—acylation, etherification, or polymer crosslinking. It isn’t just a one-trick pony. I've worked with teams that leverage this approach to anchor hydrophilic domains within water-repellent siloxane networks, boosting compatibility without giving up durability. In R&D, that small tweak in molecular makeup yields big shifts in product performance, from long-lasting adhesives to engineered elastomers. The compound’s ability to accept new side reactions has kept it in the running as more industries face pressure to cut hazardous surfactants and shift to safer, silicon-based alternatives.
This chemical carries several names across catalogs: 3-(Acetyloxy)propylmethyl-dichlorosilane, 1,3-dichloro-1-methyl-3-(O-acetyl)propylsilane, and methyl(3-acetoxypropyl)dichlorosilane show up most often. Custom syntheses under contract typically use short-hand codes or catalog references, especially in electronics and composites manufacturing. Don’t expect walk-in retailers to stock it. Most procurement flows through specialty brokers who understand niche chemical sourcing and compliance with ever-evolving transportation rules for reactive silanes.
Handle this silane with care. Any exposure to moisture generates hydrochloric acid, and that means both immediate corrosion and lasting pitting of sensitive parts around the bench. Ventilated hoods and spill kits become mandatory, and even then, you need to keep a sharp eye open for leaks. Proper training covers what happens if the bottle cracks—fast evacuation, neutralization, and cleanup, with no shortcuts. OSHA standards require documentation of exposure limits, but real-world best practice means regular air monitoring and periodic retraining. I’ve seen colleagues learn this the hard way after missing signs of acid seepage during a busy production run. Plant managers never forget the lesson: invest in good PPE and sensors, or spend far more cleaning up after a mistake.
The main attraction for 3-acetoxypropyl methyl dichlorosilane falls within advanced material manufacturing. Companies developing hybrid polymers, glass coatings, and self-assembling nanostructures appreciate the silane’s ability to introduce reactive nodes precisely. In my consulting days for the electronics sector, microchip passivation layers benefited from well-designed silane linkers that could bond strongly with oxide surfaces while still accommodating post-deposition processing. Manufacturers of adhesives and sealants tap into its chemistry for weather-resistant formulations—applying it in automotive and aerospace parts where durability and resistance to hydrolysis determine performance. Biomedical device developers and fiber-reinforced composite producers want surface modifications that survive harsh sterilization or environmental exposure, and this silane’s easy coupling plays a crucial role.
Research on functional silanes never slowed down. Recent peer-reviewed papers analyze how replacing acetate with other protecting groups impacts crosslinking speed or shelf-life stability. Some groups mix the silane with nano-fillers, crafting composites that self-heal or adapt to temperature swings. Analytical chemists dive into real-time reaction tracking, often matching machine learning with more traditional titration and spectroscopy. Every improvement demands close tracking of the outcome in the field—will the modified silane improve UV resistance? Does it help paint formulations stick better to corrosion-prone substrates? In ongoing collaborations, I see startups building out libraries of customized silanes, expanding from lab curiosity to mainstay toolkit ingredients. The challenge remains to scale up sustainable manufacturing practices without trading away product performance.
Anyone working with dichlorosilanes recognizes the risks. Early studies focused on acute exposure—burns, respiratory distress, and eye damage from acid production on contact with tissue. With improved regulation came more nuanced examinations into chronic low-level effects. Rodent assays and cell culture work suggest that most of the danger stems from a mix of HCl release and reactivity with biological nucleophiles. Modern plant operators screen air and water effluent for halide levels, knowing that spills into wastewater spell trouble for both compliance and local ecology. Regulatory agencies continue to work with universities to set exposure limits based on metabolism studies and accident reports. Responsible labs track worker exposure, rotate staff to minimize cumulative risk, and invest in process automation where the chemistry still demands hands-on touch.
Looking ahead, demand for custom-tailored silanes matches the rise of new industries that depend on hybrid materials. Sectors like flexible electronics, advanced coatings, or green energy storage look for coupling agents that can keep up with environmental and performance demands. Researchers will push for more eco-friendly syntheses, aiming to cut chlorinated by-products and ramp up process safety. Robotics and digital oversight may soon automate the most dangerous steps, minimizing direct contact and exposure. If the regulatory climate shifts harder, chemical producers may need to swap chlorosilane approaches for less hazardous analogues, or build out recovery loops to recycle byproducts. As a touchstone of functional silane chemistry, 3-acetoxypropyl methyl dichlorosilane stands to shape the interface between organic innovation and practical industrial scaling for years to come.
Anybody who has dealt with adhesives, coatings, or electronic components knows that specialty chemicals play a key role behind the scenes. 3-Acetoxypropyl Methyl Dichlorosilane gets brought in when companies look for a way to tweak surfaces for better bonding or more durability. This organosilane isn’t a household name, but it fills an important spot in industries like electronics, automotive, and construction. The formula brings together the reactivity of silanes with a structure that fits well into more complex molecules.
I remember the first time I handled a silane-based coating in a small electronics repair shop. The difference was simple but clear—components sealed better, with fewer callbacks for repair. That’s partly thanks to compounds like 3-Acetoxypropyl Methyl Dichlorosilane. In electronics, manufacturers count on it to treat surfaces and boost moisture resistance. Since fat-fingered accidents can destroy a delicate circuit, this protection matters. The technology strengthens the bond between glass, metals, and plastics. That means fewer failures and longer life for devices.
Auto makers chase lighter, tougher materials to keep up with new regulations and customer demand for better fuel mileage. Plastics, composites, and lightweight metals need to stick together under heat, stress, and vibration. Using silanes like 3-Acetoxypropyl Methyl Dichlorosilane helps make those joins stronger. It reacts with both organic resins and inorganic fillers, making sure surfaces that wouldn’t otherwise mix can stay stable. In my work with a local body shop, the difference in adhesive strength after surface treatment was night and day. The panels lined up right and stayed put, even on rough roads.
Industry values this chemical for its reactivity. Add it during the manufacturing stage, and the silyl group attaches to surfaces that need improving—think circuit boards or specialty glass. This unlocks hydrophobic properties, making it useful for products that deal with moisture, from solar panels to coated fabrics. Research in published journals confirms the reliability of silane-treated interfaces in challenging environments, boosting both safety and performance.
It’s not all upside, though. Like many silanes, 3-Acetoxypropyl Methyl Dichlorosilane demands strict care. Inhalation can irritate, and spills corrode skin. At a chemical plant I toured in the Midwest, everybody wore goggles and protective gloves around these silanes as a rule. Good ventilation, careful dosing, and clear safety training keep problems away. According to safety data, spills handled with standard absorbents reduce risks during cleanup. Green chemistry efforts are pushing for safer alternatives, but for now, careful storage and responsible disposal rank high in its use.
Silane chemistry doesn’t usually raise headlines, but it touches sustainability goals in manufacturing. Improved adhesion and crosslinking, made possible by compounds like this, allow for thinner coatings and less material waste. Fewer repairs and longer product lives translate into lower breakage rates and smaller environmental footprints. A study from the Journal of Applied Polymer Science showed that silane-treated composites often outperform untreated materials in lifecycle tests, underscoring real-world benefits.
More companies now factor in both supply chain transparency and worker safety when choosing these additives. Smart innovation and honest reporting—backed by third-party audits—make those gains stick. Every time a new application comes along, there’s a chance to select handling methods that keep both people and the planet in mind. Keeping the science sound and the operations safe ensures that advanced chemicals like 3-Acetoxypropyl Methyl Dichlorosilane can keep improving the products we rely on every day.
Anyone who’s spent time in a lab knows certain chemicals call for extra care. 3-Acetoxypropyl Methyl Dichlorosilane is one of those compounds that can catch people off guard with just how unforgiving it can be. It reacts fiercely with water, including the moisture on our skin or in our eyes, breaking down into corrosive hydrochloric acid and other byproducts you want nowhere near your body. Breathing in its vapors can wreck your lungs. Simple contact can result in burns or deeper tissue damage. This is not just theory — I’ve seen small splashes eat holes in lab coats and gloves, and it doesn’t stop there.
A solid pair of gloves means the difference between a routine job and a sprint to the safety shower. Not all gloves stop this stuff; only a heavy-duty chemical-resistant glove stands up. Nitrile often works, but check the manufacturer guide first. Always put on safety goggles with side shields or, better, a full face shield. I once watched a colleague rinse his face for fifteen minutes because he cracked open a bottle without eye protection. Even a face mask didn’t help in that case — you need a complete seal between the chemical and your skin.
Never underestimate the power of good air movement. Fume hoods make a huge impact. Opening a bottle of this silane in open air lets its vapors travel further than you might expect — and they sting the nostrils instantly. I once handled a similar compound outside a hood, just for a “quick transfer,” and I paid for it for hours. Use the hood, check its airflow, and make sure the sash sits at the right height. A room fan doesn’t cut it, and “toughing it out” can lead straight to a call with the site medic.
Check your safety shower and eyewash station before you start. Make sure your team knows where they are, and that nothing blocks access. Clean up chemicals right away; even a tiny spill dries on benches and reacts with the humidity, so you get vapors hours later. Absorbent pads meant for acids work best for cleanup. Never mix this chemical with anything else, even “safe” solvents, unless you’ve checked the literature and your protocols.
Relying on formal safety data sheets keeps you in the know, but real safety comes from conversations and drills. I remember training sessions where each person read out loud what to do in an emergency. The steps stuck when we had to do them by memory. Share war stories and tips. A missed step or shortcut can have real consequences with something reactive like this.
If you don’t respect chemicals like 3-Acetoxypropyl Methyl Dichlorosilane, you get burned — literally. You set the tone by checking your gear, keeping your bench spotless, and refusing to work when something feels off. Push back against shortcuts. Every person in a facility shares the responsibility, no matter if you’re an old hand or just starting out. Protect yourself and back up your colleagues. That’s how nobody gets hurt.
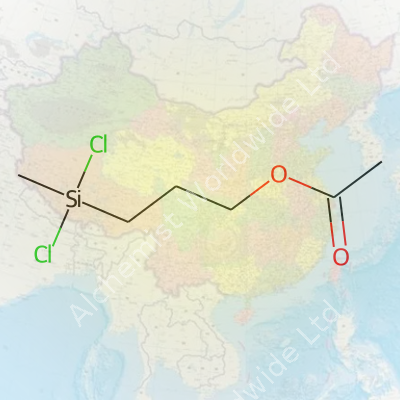
3-Acetoxypropyl methyl dichlorosilane merges both silane chemistry and acetoxy-functional organic groups in one compact structure. The name already sneaks in clues. Methyl dichlorosilane forms the core, with a three-carbon arm attached to silicon. The acetoxy group, familiar from acetic acid derivatives, anchors at the third carbon on this chain.
To picture the formula, you can start with the silane skeleton. It holds one methyl group (–CH3), two chlorines (–Cl), and a linker that brings in the rest. That linker is a three-carbon arrangement (–CH2–CH2–CH2–), finished off with an acetoxy group (–O–C(O)–CH3). It puts the whole structure together as CH3SiCl2–(CH2)3–O–C(O)CH3.
The silicon atom links with the methyl group and two chlorines, setting up a reactive core able to interact with water, alcohols, or other reactive ends. The three-carbon chain stands in as a flexible bridge, making room for the acetoxy group to stick out from the main backbone. This acetoxy tip shapes much of the compound’s behavior: it nudges the molecule toward certain reactions, gives solvents a place to grab hold, or helps it fit better into coatings or adhesives. That simple –O–C(O)–CH3 group means a lab tech or chemical engineer might look to this silane when aiming for acetoxy cross-linking.
Every tweak to a silane’s formula impacts its performance. From working in polymer cross-linking, to holding together sealants in demanding environments, these changes aren’t just numbers on a page. A group like acetoxy affects both how the silane reacts and how it resists water or heat. For engineers, knowing the structure helps isolate exactly which silane to pick when a project calls for strong bonding and flexible curing.
This isn’t just theory; I’ve seen the wrong silane cost money. In manufacturing jobs, picking a methyl-based silane instead of an ethyl variant changes the way a part handles stress tests. The three-carbon chain is not just a spacer—it gives the molecule shape and reach. If the chain were shorter, it would stunt flexibility. Some research reports have even pointed out that extending or branching out this chain can switch up how a silane sticks to surfaces.
Working with methyl dichlorosilane-based chemicals requires real respect for safety. Chlorosilanes react with water to toss out HCl gas, which calls for strong ventilation and good protective gear. The industry has moved towards emphasizing these safety steps, especially as newer workers enter the lab. Sharing experience can help: I learned early that if you don’t keep these liquids away from a damp bench, things get messy fast.
Plenty of the world’s innovation in coatings or adhesives leans on specialized silanes. As industry moves toward greener options, alternatives to chlorosilanes or acetoxy groups tend to crowd the conversation. Nothing folds perfectly into every application, but tuning molecules like this one creates tailored solutions without forcing one formula everywhere.
3-Acetoxypropyl Methyl Dichlorosilane sounds like something that rarely leaves a lab. In truth, the industry puts it into gears, coatings, and plenty of formulations. The stuff turns nasty fast if given the wrong storage or if people try to cut corners when moving it. Treating this chemical as just another bottle on a shelf could lead to ruined goods, dangerous fumes, or worse — workplace injuries that follow people for the rest of their lives.
Every seasoned tech who works with this silane will mention moisture. Water sparks a violent reaction, releasing hydrochloric acid and creating sticky messes fast. I’ve worked in facilities where a single leak from a broken seal caused alarms and rapid evacuations. Storage in well-sealed glass or high-grade stainless drums in a bone-dry warehouse can stop trouble before it ever starts. Extra care goes into checking seals for slow leaks, keeping humidity low, and locking this material in dedicated containment cabinets. Nobody wants to open a cabinet and find corrosion or weird white crystals.
There’s also a temperature sweet spot. Once, when a neighboring plant’s air conditioning failed, their supply baked under the summer sun. The heat cooked up enough pressure in containers to cause sticky valve failures and forced a costly clean-up. Cool, shaded storage keeps containers stable and workers safe.
Some might think tossing a drum onto a standard pallet and wrapping it in plastic film is good enough. That line of thinking ran through more than one facility in my early years, and I saw hazmat teams called more than once. Dedicated containers that won’t break down or corrode under acidic vapor are worth every cent. Stainless steel tanks or lined barrels cut the risk way down. I’ve met drivers who went through two sets of gloves while loading single pallets just to make sure not a drop hit their skin.
Ventilation on trucks should always be working, and explodable vapors should never be given a chance to gather. I’ve witnessed spot checks where staff sniffed for acid and quickly locked down the dock after one whiff. If a truck driver hears the phrase “secondary containment,” they know managers have done their homework — and so have regulators.
It’s never smart to trust that a new worker just reads the label, shrugs, and carries on. Onboarding in labs I’ve worked in always included gloves-on walkthroughs: how to open a drum without releasing fumes, how to suit up when a seal looks dodgy, where to go if your glove starts to itch. Strong labeling avoids the “I thought it was something else” moment. Material Safety Data Sheets shouldn’t gather dust — regular briefings save lives and prevent six-figure clean-up bills.
Transportation staff, plant workers, and even warehouse visitors need reminders. A forgotten label or a misdirected drum never stays a small problem. Investing in real training and rigid labeling doesn’t just tick a box for compliance — it lays the groundwork for trust.
A safe setup for 3-Acetoxypropyl Methyl Dichlorosilane means staying one step ahead of spills, leaks, or careless mistakes. Tight controls at every stage — from choosing the right container to double-checking training — offer more than safety for a company or a badge for an inspector. For anyone earning a living in chemical plants and warehouse floors, these rules keep the day from turning sideways. That’s worth far more than convenience or cost savings.
Many don’t hear much about 3-Acetoxypropyl Methyl Dichlorosilane beyond technical circles. This colorless to pale yellow liquid doesn’t grab attention like household chemicals, but it holds a unique spot in specialty manufacturing. Its appeal stems from how it behaves—both physically and chemically—and this has implications for safety, handling, and industrial usage.
If you ever handled or observed this compound, volatility jumps out right away. It evaporates quickly, especially in warm or open areas. Its boiling point tends to sit between 70°C to 100°C at standard pressure, which is quite low compared to larger silane compounds. You'd notice a distinct, pungent odor that warns you something reactive is at play. Viscosity remains low, so it pours easily, almost like water, which means spills happen fast if you lose focus. Its density falls below water, which brings up disposal considerations—pouring it into a drain without thought could send it floating away and causing trouble.
This silane loves to react with water. The acetoxy group and dichlorosilane unit break apart during hydrolysis, releasing hydrochloric acid and acetic acid vapors. In my lab days, even a small splash into a moist beaker sent acrid fumes swirling—goggles and gloves became non-negotiable. Uncontrolled contact with air humidity can cause corrosion to metal surfaces and damage pH-sensitive equipment.
Flammability doesn’t dominate concern as much as moisture sensitivity. Still, you can never ignore the byproducts: hydrogen chloride gas stings eyes and lungs, acetoxy groups release their own sharp scent. If you store it, glass containers with airtight caps hold up better than plastic, which hydrolyzes and deforms under acidic assault over time.
Those low boiling points combine with moisture reactivity to shape both storage logistics and workplace hazards. Facilities keep this silane locked away from humid rooms. I’ve seen small leaks lead to steamy vapor clouds within minutes, creating immediate evacuation needs. Companies teach their teams about this unpredictability, pointing out real case studies where things went wrong.
On the upside, these reactive traits play into precise chemical synthesis. The molecule transfers acetoxypropyl and methyl groups to other compounds, tailoring surfaces or producing resilient siloxane bonds. Electronic sealants, coatings, and specialized adhesives benefit from these features, allowing products to stand up to tough conditions. That quick hydrolysis acts like a built-in setting agent in moisture-cure systems, turning a liquid into a solid protective layer.
Mismanagement carries consequences—from minor irritations to bigger threats like toxic gas buildup. Staff training, proper PPE, and real attention to local exhaust ventilation have cut down incident rates in many labs. On the environmental side, local regulations prevent draining into waterways. Neutralizing waste with sodium bicarbonate or similar bases before disposal reduces risk.
Process upgrades, like better air sensors and spill-proof dispensing tools, make life easier for workers. Manufacturers continue modifying silane molecules, searching for versions that present fewer hazards but still work reliably. Regular lab reviews and incident reporting back up these changes, complementing better product labeling and public data sheets.
| Names | |
| Preferred IUPAC name | 3-(Acetyloxy)propyl-dichloro-methylsilane |
| Other names |
3-(Acetyloxy)propylmethyldichlorosilane Gamma-Acetoxypropylmethyldichlorosilane 3-Acetoxypropyl(methyl)dichlorosilane |
| Pronunciation | /ˈθriː əˈsiːtəksi ˈproʊpəl ˈmɛθəl daɪˈklɔːr.oʊˌsaɪleɪn/ |
| Identifiers | |
| CAS Number | [17863-97-7] |
| 3D model (JSmol) | `3d:13-2(3)1-6(4,14)5-7-8-10-12-9-11-7/h1H2,5H2,4H3` |
| Beilstein Reference | 1576422 |
| ChEBI | CHEBI:131212 |
| ChEMBL | CHEMBL3724686 |
| ChemSpider | 145624 |
| DrugBank | DB16265 |
| ECHA InfoCard | 03b0f3e1-b3d3-48b0-9750-86b290220a26 |
| EC Number | 4253-35-6 |
| Gmelin Reference | 88400 |
| KEGG | C19274 |
| MeSH | Dichloromethylsilane |
| PubChem CID | 86712083 |
| RTECS number | TX9625000 |
| UNII | CNW3R1VD8D |
| UN number | UN3265 |
| Properties | |
| Chemical formula | C6H11Cl2O2Si |
| Molar mass | 238.17 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Pungent |
| Density | 1.16 g/mL at 25 °C(lit.) |
| Solubility in water | Reacts with water |
| log P | 0.7 |
| Vapor pressure | 0.5 mmHg (25 °C) |
| Acidity (pKa) | 13.2 |
| Basicity (pKb) | 6.6 |
| Magnetic susceptibility (χ) | -65.0e-6 cm³/mol |
| Refractive index (nD) | 1.443 |
| Viscosity | 3 cP (25 °C) |
| Dipole moment | 3.24 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 489.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H314: Causes severe skin burns and eye damage. |
| Precautionary statements | P210, P233, P235, P240, P241, P242, P243, P260, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P312, P321, P331, P337+P313, P363, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 3-1-2-W |
| Flash point | 77 °C |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 1780 mg/kg |
| NIOSH | BWC82250 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | IDLH: Unknown |
| Related compounds | |
| Related compounds |
Trimethylacetoxysilane Vinyltriacetoxysilane 3-Aminopropylmethyldichlorosilane 3-Glycidyloxypropylmethyldichlorosilane Methyltrichlorosilane |