
Chemists have always scouted for ways to bridge the gap between organic matter and inorganic substrates, and silane coupling agents have played a major part. Back in the mid-20th century, researchers began to unlock the potential of organosilicon compounds for their unique way of linking two chemically different worlds. Among these, 3-Acetoxypropyltrimethoxysilane landed on the scene not by accident but as part of the ongoing hunt for more stable, reactive, and effective silanes. Its emergence marked a shift toward functional groups that could bring versatility to surface treatments or polymer chemistry. This compound let material scientists experiment with adhesion and compatibility for things like glass fiber composites, coatings, and plastics, building on the shoulders of earlier silane studies that often relied on alkoxy or amino groups.
3-Acetoxypropyltrimethoxysilane stands out from the crowd of silane agents for its balance of reactivity and handling convenience. Its design includes an acetoxy group sitting on a propyl chain, which connects to a silicon atom holding three methoxy groups. This combination lets it act as a bridge between hydrophobic organics and hydrophilic surfaces, expanding its use in everything from adhesives to sealants. Instead of being limited to just one purpose, this compound gets drafted into jobs that need surface modification, functionalization, and better bonding between composites and substrates.
On the shelf, 3-Acetoxypropyltrimethoxysilane usually appears as a clear, colorless, and mobile liquid. Its boiling point lands well above room temperature, so storage and transport conditions stay manageable. The acetoxy group introduces some hydrolysis sensitivity, but not at a rate that stumps practical use. The methoxy groups attached to the silicon atom are ready to interact with water or alcohols, leading to silanol formation and subsequent bonding with hydroxyl-rich surfaces. Its molecular weight comes in at about 236.32 g/mol. Under normal lab conditions, it doesn't give off much odor, which lowers concerns about strong fumes, and its solubility profile leans towards organic solvents like alcohols, acetone, and to a limited extent, water before hydrolysis starts.
Most suppliers deliver 3-Acetoxypropyltrimethoxysilane at a purity of over 97%, which lines up with the needs of industrial processes that don't tolerate excess by-products. Standard labeling includes hazard ratings for skin and eye irritation, as well as guidance on proper ventilated usage. Packaging typically involves sealed, moisture-tight containers ranging from small glass bottles for research labs to drums for large-scale manufacturing. Information sheets highlight its reactivity with water, emphasizing proper storage away from humidity, and call attention to flammability similar to other low-molecular-weight organosilanes.
Synthesis starts with a straightforward path: propylene glycol reacts with acetic anhydride, adding the acetoxypropyl chain, while subsequent treatment with trimethoxysilane in the presence of a catalyst forges the silicon-oxygen bonds. This process usually gets fine-tuned for yield and selectivity by tweaking temperature, pressure, or catalyst loading. Labs dedicated to pilot-scale production pay extra attention to controlling water presence, since excess moisture can trigger premature hydrolysis, ruining the batch or leading to difficult cleanups. Careful distillation and purification trim away side products, giving a final product with the reliability demanded by circuit board manufacturers, glass treatment shops, and research outfits tinkering with hybrid polymers.
The three methoxy groups on the silicon atom give 3-Acetoxypropyltrimethoxysilane a reactive edge for crosslinking and grafting onto surfaces. Exposed to heat or acidic water, these groups hydrolyze and form silanols. Once present, the silanols can condense onto glass, metal oxides, or even cellulose, resulting in strong, covalently bound layers. The acetoxypropyl tail presents an interesting handle for further modification; in laboratories, it sometimes takes part in transesterification, or acts as a protected functional group for downstream chemistry. This set of reactions turns the compound into a molecular connector, not just a surface coat.
3-Acetoxypropyltrimethoxysilane pops up under different names from one catalog to another. Some common aliases include 3-(Acetyloxy)propyltrimethoxysilane, Trimethoxy[3-(acetoxy)propyl]silane, and simply S116 or KBM-573 in industrial shorthand. Each label points to the same core structure, giving buyers and scientists a way to navigate across suppliers and publications. The diversity of names sometimes causes mix-ups, so careful checking against CAS registration (CAS No. 4663-53-0) cuts down on ordering mistakes and keeps projects on track.
Handling 3-Acetoxypropyltrimethoxysilane calls for a level head and respect for both chemistry and personal safety. Skin and eye contact brings stinging or worse, while inhalation brings risk of respiratory trouble. Good labs use gloves, goggles, and designated fume hoods, even for quick experiments. Transport regulations echo those of other organosilanes – keep away from open flames, store in dry, locked chemical cabinets, and double-check all connections and seals to prevent accidental leaks. Waste disposal involves neutralization, then routing through permitted chemical waste channels, dodging environmental harm or dangerous buildup in local waterways. Over the years, chemical safety boards have tightened rules, so up-to-date training and regular safety reviews make for smoother, safer operations.
3-Acetoxypropyltrimethoxysilane's versatility shows in its industrial reach. In composites manufacturing, engineers use it to anchor resins to glass or mineral fillers, solidifying everything from automotive parts to circuit boards. In construction, it fortifies sealants and adhesives, extending lifespan and resisting moisture-driven decay. Its capacity to tailor surface energy transforms standard glass into substrates that accept paints, inks, or electronic films, shaking off the frustration of poor adhesion or peeling. Paint and coating formulations depend on it for better scratch resistance and longer weatherability. Over in textile finishing, this compound assists in binding functional finishes onto fibers, showing just how far a well-designed silane can stretch across sectors.
Universities and industrial R&D shops take an interest in 3-Acetoxypropyltrimethoxysilane for its role as a gateway to next-gen hybrid materials. Research projects target ways to tweak its reactivity, aiming at greener processes or smarter, adaptive surfaces. Academic papers dig deep into how this silane interacts with nanoparticles, hoping to unlock new classes of functionalized fillers for medicine, filtration, or energy storage. Patent filings suggest that adding different groups to the basic structure opens doors to block copolymers or responsive coatings. In my own time at a polymer lab, chasing new adhesive chemistries, adding a dash of 3-Acetoxypropyltrimethoxysilane sent pull test results soaring, turning once-impossible projects into practical, scalable jobs.
Health impact remains a heavy thread in the conversation about silanes. Regulatory bodies demand up-to-date toxicity screens before approving chemicals for commercial use. Studies have shown that while acute exposure to 3-Acetoxypropyltrimethoxysilane brings irritation, the bigger concern surrounds chronic inhalation and environmental persistence. Animal studies get cited in safety assessments, and newer in vitro tools scan for DNA damage and metabolic breakdown rates. Like many reactive organosilanes, its breakdown products include methanol, known for its own toxicity risks, which puts safe handling ahead of all development. Data still pours in, with each new result sharpening understanding and sometimes shifting workplace guidelines. These efforts ensure the chemistry boom does not outpace worker and environmental health.
Down the road, 3-Acetoxypropyltrimethoxysilane seems set to grow its presence as industries lean into lightweight composites, smart coatings, and energy-efficient buildings. Advances in formulations that tame its hydrolysis speed or reduce greenhouse gas impact promise to widen its acceptance, even in fields that once passed over silanes for more familiar binders. Sustainability challenges push researchers to look for silane agents with milder synthesis routes, easier recyclability, and less hazardous by-products. Green chemistry angles could see the acetoxypropyl group swapped for bio-derived alternates, while digital modeling tools may design even more efficient coupling agents. My own view is that firms investing in safer handling systems, better worker protections, and creative recycling solutions will lead the charge, carving out value beyond the chemistry itself and cementing a responsible legacy for the next generation of material scientists and entrepreneurs.
3-Acetoxypropyltrimethoxysilane might sound like a mouthful, but in labs and on shop floors, it usually shows up as an all-purpose helper in making things stick, last, and survive tough conditions. This chemical jumps front and center in the business of coupling agents—basically, it’s a bridge connecting materials that don’t naturally want to play well together. You’ll hear about it in the context of coating glass or modifying a plastic, but that’s just the start.
Imagine you’re building windows for high-rises or windshields that need to last for years without fogging, peeling, or letting water sneak through. Glass and plastics don’t bond easily, and untreated surfaces can let moisture or air damage set in. 3-Acetoxypropyltrimethoxysilane shows up as a treatment step, prepping these surfaces. The chemical reacts with the glass to form a strong, lasting bond, while also connecting to the chosen adhesive or polymer. Factory workers spray or dip glass panels in a diluted solution, let it dry, and then the next coating grabs on much tighter.
On the manufacturing side, it’s about cutting down on failed seals and lost customer returns. That’s cash saved, trust built, and less waste.
Fiberglass boats, printed circuit boards, even industrial panels—these products rely on a mix of fibers and resins that don’t just meld together by luck. Anyone who’s ever tried patching a fiberglass pipe learns quickly the bond needs more than Elmer’s glue. In composite work, 3-Acetoxypropyltrimethoxysilane treats the fibers before they meet the resin. This leads to fewer cracks, less water damage, and longer life for the end product. Quality control teams notice a jump in performance, and customers see gear that holds up longer.
If you’ve worked assembling circuit boards, you notice how even small flaws in insulation or adhesion turn into heat buildup, shorts, or early failure. Silane coupling agents like this one play a hidden role. Process engineers add it in the mix to boost adhesion between the silicon surfaces and insulating layers. When connections stay stable, especially in critical devices, the board resists heat, water, and rough handling from daily use.
Adopting 3-Acetoxypropyltrimethoxysilane as a standard step in production lines doesn’t just improve bonds. It also saves money and material in the long run. The more reliable the connection between glass, composite, or electronic components, the less downtime for rework. I’ve talked with production managers who count on it to keep their rejection rates in check, especially as customer expectations keep rising.
As manufacturers shift to lighter, more complex materials—think electric vehicle shells or advanced medical equipment—the need for proven, consistent coupling agents matters more than ever. 3-Acetoxypropyltrimethoxysilane fits that bill, balancing reliability with cost, safety, and efficiency. Companies investing in research want better performance at lower voltages, tougher windshield coatings, or faster-curing adhesives. Feedback from the floor helps fine-tune how the chemical gets used, keeping pace with new challenges in production and repair settings.
As someone who’s seen production slowdowns from bad adhesion or failed composites, it’s clear why chemicals like this matter. They don’t just stay in the lab—they help shape products people count on every day.
3-Acetoxypropyltrimethoxysilane has a straightforward structure for folks who know their way around a chemistry set. The chemical formula is C9H20O5Si. What you see here is a combination that industry workers, lab analysts, and materials scientists depend on across a handful of significant applications. Understanding what that string of letters and numbers represents helps us appreciate its impact outside chemical shelf labels.
Products like adhesives, coatings, and sealants don't just appear out of thin air. Manufacturers rely on silane coupling agents to marry materials that wouldn’t ordinarily stick together. The acetoxypropyl part does its job by improving compatibility between organic and inorganic surfaces. That helps when joining glass fibers and resins or when adding durability to protective coatings. The trimethoxysilane end lets the molecule react with moisture, giving a solid bond and more resilience against weathering.
Back in the lab, chemists working on composite materials look at the silane category as a solution for persistent adhesion problems. Without molecular bridges like C9H20O5Si, things fall apart—literally. Over the years, I've watched teams cycle through one fomulation after another. When they get the silane blend right, they see paint stick, composites hold, and glass meet polymer without flaking off a few months in. It saves time, cash, and a lot of headaches down the line.
Chemicals that sound technical can unsettle anybody without a background in science. Issues of health, safety, and environmental handling hit close to home for people working on factory floors or in small businesses mixing their own solutions. Industry studies flag certain silanes as eye and skin irritants, so wearing gloves and goggles remains pretty standard advice. Proper ventilation keeps inhalation risks low. It's not enough to drop safety data sheets onto a desk. Real-world training, clear signage, and hands-on guidance help keep workplaces safe.
Sustainability plays a bigger and bigger role in how companies source, use, and dispose of chemicals like 3-acetoxypropyltrimethoxysilane. Waste management doesn't make the news often, but cutting down on runoff and improving recycling of spent materials feeds directly into healthier rivers, safer soil, and better air. Regulators want cleaner production, and businesses smart enough to adapt wind up safer and stronger as a result.
People who tackle challenges with silanes often juggle multiple priorities. Customers want reliability; regulators demand safer practices. Universities push for greener chemistry each year. The chemical formula C9H20O5Si might be one line on a spec sheet, but it sits behind years of research, real-world performance, and day-to-day problem solving. By making smarter choices, investing in employee safety, and planning for end-of-life recycling, those handling these chemicals can stretch benefits further—keeping workplaces resilient, communities safer, and products built to last.
3-Acetoxypropyltrimethoxysilane belongs to the world of organosilanes. Folks use this compound in coatings, adhesives, and glass treatments. It’s part of the backbone for materials that need a little extra performance. If you’ve ever dealt with silanes, you know the warnings on the label are not for show. This liquid reacts, breaks down, or releases toxic fumes if storage strays even a little from best practice.
3-Acetoxypropyltrimethoxysilane pulls moisture from the air. Once water vapor gets in, hydrolysis happens. You end up with acetic acid and a sticky mess. That’s not only a waste of chemicals, but a safety risk. From personal experience, even small leaks lead to weird odors and ruined drums. Storing this chemical in airtight drums with tight seals will help. Metal drums with lined interiors work best, since unlined steel can trigger other reactions. Polyethylene jugs can degrade with certain chemicals, so make sure the container matches up with the supplier’s recommendations.
Heat speeds up trouble with 3-Acetoxypropyltrimethoxysilane. Keep these drums in a cool spot, below 30°C. A shaded corner of the warehouse works fine as long as air moves freely. Chemical storerooms sometimes start baking during the summer, especially if ventilation’s overlooked. Fans go a long way to keeping that temperature down. Direct sunlight not only warms up the product, it also brings UV into the picture, which can kickstart breakdown of silane bonds. I’ve walked into storage areas to find cracked containers and lost inventory simply because someone stacked them next to a window.
Moisture sneaks in during drum transfers or whenever containers get opened. Always close the cap firmly straight after dispensing. If the warehouse gets humid, silica gel packs or other desiccants in the storage area absorb excess moisture. I once watched a shipment go bad in just a few weeks because caps weren’t tightened after sampling, and Baltimore gets muggy in spring. Use nitrogen to blank the headspace if possible. That’s a trick many chemical handlers use: a quick shot of dry nitrogen keeps air and moisture out without complicated equipment.
Mixing chemicals is rarely a good idea. Store silanes apart from acids, bases, and oxidizers. Cross-contamination leads to unexpected reactions. Clear labels and diligent stock rotation make a difference in identifying spills or leaks early. In my experience, barcode tracking and routine drum checks save far more product than letting containers gather dust in the back of the warehouse. Safety Data Sheets posted right near the storage rack give everyone a quick reminder of emergency steps if something goes wrong.
Cutting corners with storage means higher costs down the road—lost product, health risks, even regulatory trouble. Proper storage not only protects workers but keeps the chemistry reliable. Quality issues often trace back not to formulation errors but poor storage habits. Anyone who’s handled batches that smelled “off” knows how fast an avoidable error can turn into a waste disposal headache. Handling 3-Acetoxypropyltrimethoxysilane with respect pays off every time in safer, cleaner workspaces and predictable quality on the production line.
3-Acetoxypropyltrimethoxysilane, a mouthful to say and even more to handle, sits among those chemicals that help make coatings, adhesives, and sealants stronger and more weather-resistant. On a factory floor or in a lab, some workers have handled this silane without much fuss, but one moment of carelessness can pack enough punch to cause real trouble.
A strong vinegary odor sometimes rides the air when opening a drum. People who have handled 3-APTMOS know how it can sting if the liquid splashes on bare skin. Eyes feel the burn, and inhaling the vapor causes throat irritation and coughing. Chronic skin contact leaves rough spots, even chemical burns. Breathing the fumes day in and day out? Not great for anybody’s lungs. Safety data sheets point out the risks, but practical experience always hammers the lesson home.
Anyone who thinks a pair of thin cotton gloves can stand up to this stuff should think again. Chemical-resistant gloves—think nitrile, not latex—do a better job. Proper goggles and face shields act as the front line against unexpected splashes. Lab coats or disposable coveralls give extra protection, especially when pouring large volumes or working in tight quarters. Boots should resist chemicals instead of soaking things up. These steps helped workers keep their skin and eyes out of trouble, whether prepping a reaction vessel or just cleaning up spills at the end of a shift.
Opening a container in a closed-off storeroom leaves fumes hanging in the air. Some shops keep their silane operations next to an open door, but that’s not always enough. Locating the process under a fume hood or installing local exhaust pulls vapors away from faces and out of the breathing zone. Monitoring air quality helps spot ventilation problems before anyone starts coughing. Nobody likes a persistent chemical odor hanging around—air movement keeps things fresh and avoids surprises.
A few stories stick with workers who have been around chemicals for years. One day a shop worker stacked a 3-APTMOS drum under a leaky air conditioner, and it rusted through faster than anybody expected. These silanes don’t belong near water or acids. Metal corrosion and chemical reactions kick off easily, so picking plastic or stainless steel makes a difference.
Drums and bottles stay upright, away from heat or sunlight. The labels never come off, and nobody transfers the liquid between containers without a tight seal and a working pump. Paper towels and rags tossed in open trash cans start to smell, so keeping a special solvent bin for waste goes a long way. It doesn’t take much to cause a problem, but careful habits limit the mess.
Every site needs an eyewash station and safety shower within reach. Running to cold water within ten seconds beats trying to remember the number for medical help. Quick decontamination matters, whether someone splashed their shirt or caught a mist in the eye. Emergency plans make sense before trouble begins, not after.
Safety isn’t a box to check—it comes from watching out for one another. Good habits, solid gear, and real practice go hand-in-hand with training. People take pride in working with their hands and keeping coworkers out of harm's way. Regular review and honest talk about near-misses make everyone sharper and keep the line running strong.
Every chemist working with silane blends hits the same roadblock—different chemistries don’t always play nice. 3-Acetoxypropyltrimethoxysilane often pops up thanks to its role as a coupling agent, especially when plastics or composites meet glass or metal. Lab benches fill with options, but mixing this silane with other types such as amino, epoxy, or vinyl functional silanes doesn’t guarantee a seamless result. Each batch becomes a new test. It sometimes feels a lot like cooking: add the wrong ingredient, and the whole dish changes.
Functionality directs the show. 3-Acetoxypropyltrimethoxysilane brings an acetoxy group. This group sits at a sweet spot—it quietly hydrolyzes in water, giving off acetic acid and exposing a reactive silanol. In a mixed silane system, not every functional group behaves nicely when acetic acid releases. Amino silanes, for instance, can struggle in acid, leading to slower reactions. Over time, I’ve seen formulas clump unexpectedly or fail to bond strongly. Rushing to blend these materials never brings happy surprises. So, anyone using acetoxy- and amino-functional silanes together knows to check their pH and keep a close eye on immediate gelation or poor film performance.
Mixing silanes gets messy fast. In my own experience, vinyl or epoxy silanes keep their heads in most mixtures. But 3-Acetoxypropyltrimethoxysilane brings its own timeline for hydrolysis. Pour them together, and a race begins: acetoxy groups break down at different rates than others, often shifting the phase or even causing some ingredients to separate before curing even starts. There’s a reason tech sheets—if anyone bothers to check them—warn about sudden precipitation or viscosity jumps mid-mix. This isn’t just paperwork; it’s damage control. I’ve worked on floor coatings where an off-the-shelf blend caused streaks and weak edges. Nobody likes explaining to customers why their seams are peeling up or why their glass-fiber composite lost adhesion strength after only a few months.
Experience teaches the hard lessons. Stable silane blends rely on controlling water, pH, and sequence. Tossing all silanes into one pot risks hydrolysis gone wild, poor shelf stability, or unpredictable crosslinks in end products. Those who run pilot batches before scaling up often catch trouble before thousands of kilos hit the plant. I’ve stood up nights trying to track down a haze in cured films. Turns out, swapping a trifunctional silane for a 3-acetoxypropyl one kicked off early gel formation because local water content shifted and acetoxy reacted faster than expected. Lab notes saved the day, not any fancy simulation.
Trial runs and compatibility charts only go so far. Real improvements come from clear communication between R&D, suppliers, and end-users. I’ve learned to demand technical support from manufacturers before risking expensive feedstocks. Some newer manufacturers supply premixed systems where 3-acetoxypropyltrimethoxysilane works alongside other silanes, but even here, small-scale application tests matter. Tweaking pH with buffers or playing with the order of addition can fix looming problems. Small details—lab humidity, exposure time, stirring rate—turn out to make a big difference.
Years of hands-on formulation taught me that mixing silanes, especially ones with distinct hydrolysis paths like 3-acetoxypropyltrimethoxysilane, is never a copy-paste job. Each formula acts differently, and only rigorous lab work reveals the right path for success in the field.
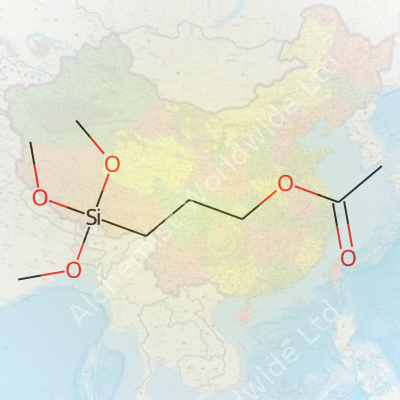
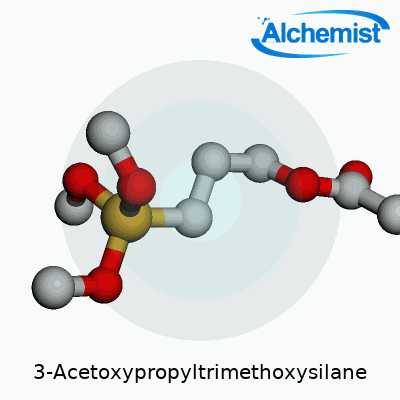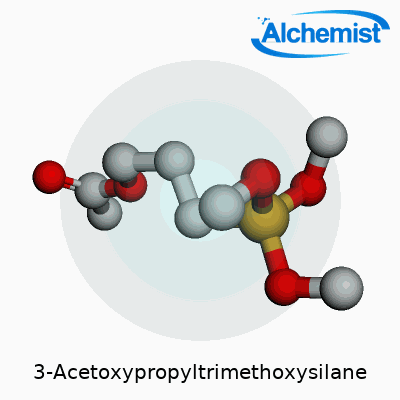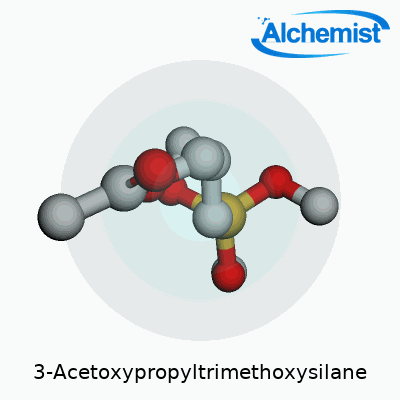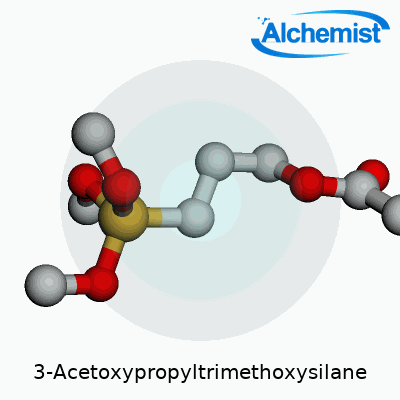
| Names | |
| Preferred IUPAC name | 3-(Trimethoxysilyl)propyl acetate |
| Other names |
3-(Trimethoxysilyl)propyl acetate 3-Acetoxypropyltrimethoxysilane Acetoxypropyltrimethoxysilane Acetoxypropylsilane Trimethoxy[3-(acetoxy)propyl]silane |
| Pronunciation | /ˈæ sə ˌtɒk si ˈproʊ pɪl traɪˌmɛθ ɒk si ˈsaɪ leɪn/ |
| Identifiers | |
| CAS Number | [3069-29-2] |
| Beilstein Reference | 3462098 |
| ChEBI | CHEBI:85058 |
| ChEMBL | CHEMBL1544970 |
| ChemSpider | 56609 |
| DrugBank | DB14696 |
| ECHA InfoCard | ECHA InfoCard: 100.066.219 |
| EC Number | 245-366-4 |
| Gmelin Reference | 1162219 |
| KEGG | C19229 |
| MeSH | C092106 |
| PubChem CID | 114625 |
| RTECS number | VV9275000 |
| UNII | QX9N50XJA5 |
| UN number | UN3334 |
| Properties | |
| Chemical formula | C8H18O5Si |
| Molar mass | 234.32 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 1.06 g/mL at 25 °C (lit.) |
| Solubility in water | Soluble in water |
| log P | 0.3 |
| Vapor pressure | 0.1 mmHg (20 °C) |
| Acidity (pKa) | pKa ≈ 16 (estimated for the acetoxy group) |
| Basicity (pKb) | pKb = 3.6 |
| Magnetic susceptibility (χ) | -62.5×10^-6 cm³/mol |
| Refractive index (nD) | 1.4150 |
| Viscosity | 2 cP |
| Dipole moment | 3.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 576.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H318 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P302+P352, P305+P351+P338, P312, P337+P313, P403+P235 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 85 °C |
| Autoignition temperature | Autoignition temperature: 245 °C |
| Lethal dose or concentration | LD50 Oral Rat 7,360 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral Rat 8025 mg/kg |
| NIOSH | GV2875000 |
| PEL (Permissible) | Not Established |
| Related compounds | |
| Related compounds |
3-Chloropropyltrimethoxysilane 3-Glycidoxypropyltrimethoxysilane 3-Mercaptopropyltrimethoxysilane 3-Aminopropyltrimethoxysilane 3-Cyanopropyltrimethoxysilane |