
The journey of (3-Acryloxypropyl)Methyldimethoxysilane stretches back to the advancement of organosilicon chemistry in the mid-twentieth century. Scientists first looked at silane coupling agents as a bridge between organic and inorganic worlds, aiming to toughen up composites and adhesives. Research interests grew right along with the plastics boom, pushing industry leaders and chemists to fine-tune molecules that toughen surfaces, bind polymers, and create longer-lasting finishes. By the ‘80s, as composite materials became more common in construction and electronics, silanes like this one started to take center stage in efforts to push the boundaries of surface modification and crosslinking.
(3-Acryloxypropyl)Methyldimethoxysilane—sometimes known under trade names like Momentive’s Silquest A-174 or Shin-Etsu’s KBM-5103—offers a fusion of acrylic and silane chemistry. It brings together the reactivity of acrylate groups with the hydrolyzability of silicon methoxy moieties, producing a molecule that docks on both organic polymers and inorganic substrates. Labs and plants keep turning to it for its ability to anchor coatings to glass, mineral fillers to resins, and to deliver abrasion resistance. Manufacturers prize its versatility, using it in everything from wind turbine blades to dental composites.
This silane compound comes as a clear, colorless or faintly yellow liquid, delivering a faint odor that hints at both acrylates and alcohols. Its molecular weight hovers around 234.34 g/mol, with a boiling point above 100°C, thanks to the stability of its siloxane backbone. Its density sits at about 1.06 g/cm3 at room temperature. As for solubility, it dissolves in common organic solvents while reacting quickly with water to yield silanols and methanol. That hydrolysis is no small matter—left in moist air, the liquid soon thickens and bonds to any nearby glass or cellulose.
Industrial shipments always display CAS number 31024-56-3, and labels flag hazards like skin irritation, eye irritation, and flammability. The recommended purity runs 97% or higher, often tested through GC or NMR analysis. Containers must keep air and water out because even a trace of humidity triggers gelation. I’ve seen storage rooms with strict 4–25°C temperature controls to avoid spoilage; even a brief opening of the drum can let in enough moisture to spell trouble. Detailed safety sheets advise PPE, splash goggles, and plenty of ventilation on the shop floor.
Manufacturing plants typically start with (3-chloropropyl)methyldimethoxysilane and acrylate it by transesterification with acrylic acid, sometimes using a base or tin catalyst. The process produces methanol, which requires careful removal to keep the yield high and the product pure. Downstream, facilities distill the mixture under reduced pressure. My experience watching these reactions up close highlights how much patience and vigilance the operators bring to the job—just a few slips, and the acrylate polymerizes, gumming up reactors and pipelines.
The acrylate group opens up so many doors for free-radical polymerizations, both in emulsion and bulk reactions. Researchers routinely graft this silane onto polymers, crosslinking with styrene, butadiene, or vinyl monomers to make weather-resistant rubbers and adhesives. On the other end, the methyldimethoxysilane group hydrolyzes in the presence of water, forming silanols that bond strongly to silica, glass, or mineral surfaces. These silanols then condense, anchoring firmly to the substrate. My lab colleagues often marvel at how small tweaks in pH or solvent can steer the entire process toward a brittle film or a flexible network.
You’ll find it listed as A-174 Silane, KBM-5103, Methacryloxypropylmethyldimethoxysilane, and, in technical catalogs, as 3-(Methacryloyloxy)propylmethyldimethoxysilane. Chemical suppliers love their codes, often using in-house designations for specific batches, which sometimes causes confusion for buyers sorting through competing quotes and technical datasheets.
Anyone who has spent time in a production hall knows the hazards with silanes. Eye contact burns and skin reddens within minutes if there’s no protection. Inhalation may lead to headaches or respiratory irritation, and, as some accident reports remind us, the liquid’s low flash point puts sparks and open flames completely out of the question. Standard practice means respirators for high concentrations, chemical gloves, and constant training refreshers on emergency spills and containment. European REACH and American OSHA rules spell out exposure limits and require yearly audits; smart suppliers deliver robust SDS (safety data sheets) attuned to local requirements. Fire drills in real facilities show how fast leaks or releases can escalate, spurring sensible investments in scrubbers and alarms.
What strikes me most about this silane is how it adapts to different industries. Composite manufacturers treat glass fibers with it, boosting adhesion to polyester and epoxy matrices. In the automotive world, it appears in paints and coatings, forging bonds between clear coats and plastic fenders, or between primers and difficult metal alloys. Adhesive developers blend it into formulas for building sealants and dental filling materials, using the dual reactivity to peg filler particles to flexible polymer backbones. Electronics companies use thin films of this compound to anchor photoresists on silicon wafers during microchip production. I’ve spoken with engineers who rely on it for microfluidic devices and biosensor assembly, where surface stability can make or break a breakthrough.
University groups and corporate labs continue to tweak reaction conditions and molecular design, seeking ways to make this silane play nicer with eco-friendly resins and biopolymers. New research looks at the effect of the silane structure on the mechanical properties of composites, especially where high humidity, salt spray, or dramatic temperature swings threaten performance. Projects in dental science investigate biocompatibility and water resistance for restorative materials. Coatings experts have explored adding pigments or antistatic agents directly to the silane, aiming for layered functionalities in one quick processing step. The R&D pipeline remains crowded with testing protocols—tensile testers, thermal cyclers, UV-exposure boxes—each revealing yet another facet of what this versatile molecule can offer.
Early toxicology screens flagged skin, eye, and upper respiratory irritation as meaningful risks, but the long-term effects of chronic exposure still invite deeper investigation. The methacrylate fragment carries a history of sensitization and occupational allergies, pushing many labs to invest in improved air scrubbing and real-time monitoring. Rodent studies suggest oral LD50 values fall in a moderate range, but firm epidemiological links in humans remain scarce. Workplace safety audits advise rotation of staff, regular serum testing for allergic sensitization, and keeping a close eye on shipping and handling routes. Environmental tests have shown moderate biodegradation, but breakdown products must still be tracked to confirm no build-up in groundwater or bioaccumulation in wildlife.
Looking ahead, demand for advanced materials won’t slow down. Manufacturers keep hunting for stronger wind blades, lighter vehicles, and tougher, stain-resistant coatings, all of which rely on clever chemistry at the molecular interface. Improvements in green chemistry, catalysis, and 3D printing present new opportunities for silanes. Industry will likely see more silanes tweaked for compatibility with bio-based polymers and recyclable resins. New regulatory frameworks, especially those targeting microplastics and VOC emissions, could push suppliers to rework formulations for lower toxicity and safer use. The chance for next-generation electronics, wearable sensors, and medical devices also keeps the spotlight on (3-Acryloxypropyl)Methyldimethoxysilane. I see no end to the questions—and that’s exactly what makes research on this compound so energizing.
Paint and coatings manufacturers look for ways to toughen surfaces against wear and weather. (3-Acryloxypropyl)Methyldimethoxysilane steps in here. This organosilane acts as a “bridge” between organic resins and inorganic pigments or fillers, creating solid bonds between both worlds. As a result, paint films gain better scratch resistance, improved adhesion to glass or metal, and a stronger grip in tough environments. Standard acrylic or epoxy paints just can't match up when it comes to hanging onto glass or tile. With this molecule, it's possible to upgrade old formulas without adding extra processing steps.
High-strength composites pop up everywhere, from bicycle frames to the body panels of electric cars. Their trick is combining a stiff substrate, often a mineral or glass fiber, with a flexible polymer matrix. Connecting these two parts isn’t easy. Most fibers are full of silanol groups. (3-Acryloxypropyl)Methyldimethoxysilane addresses the mismatch head-on by grafting its reactive acrylic side to the plastic and its silane end to the glass. This method tightens the interface, so parts don’t delaminate or lose their shape under stress. The real payoff: lighter parts that last longer and don’t give up under repeated impacts.
Modern industries often run into the need for glues that can stick to nonporous, hard-to-hold materials. Traditional adhesives struggle with smooth surfaces, especially glass or glazed ceramics. Adding this silane helps make sure there’s a chemical grip, not just a physical one. Construction and electronics both benefit—think insulated glass, touchscreens, or even weatherproof seals on LED lamps. According to published technical reports, bond strengths often double compared with regular acrylic-based glue alone. I’ve seen this first-hand on renovation projects, where certain “impossible” glass joints only held up after this additive got used.
As 3D printing grows, especially with UV-curable resins, print quality and durability matter more than ever. In these resin systems, (3-Acryloxypropyl)Methyldimethoxysilane acts as a crosslinking agent. Its acryloxy group polymerizes quickly under UV, forming a dense, interconnected network. This network results in prints that resist chipping and stand up to repeated flexing. Medical device makers and engineers working on custom parts rely on this property for product safety and reliability. Successful technical studies, such as those from American Chemical Society journals, point to better print accuracy and longer part lifespans thanks to this small but mighty ingredient.
Over the past decade, stricter environmental rules have forced companies to rethink crosslinking agents and adhesion promoters. Many traditional additives release volatile organic compounds or require harsh reaction conditions. This particular silane, by contrast, hydrolyzes under mild conditions and doesn’t leave behind problematic residues in most applications. Switching to it often means lower emissions and safer work sites, which lines up with what regulators recommend and what workers deserve. As someone who’s been on shop floors and in labs, I’ve found that safer processing pays off in both health and paperwork.
Potential Solutions to BarriersSome challenges remain, including the need for proper handling: the compound reacts with moisture, so tight packaging matters. Training staff and keeping storage areas dry helps. Manufacturers who keep quality testing in-house catch problems early and deliver better products to clients. Ongoing research looks at recycling and recovery, aiming to keep waste low and value high. By sharing knowledge between companies and universities, the field keeps evolving—this is what drives lasting improvements across industries.
Every product, whether it’s medicine, food, or a cleaning agent, comes with a set of storage instructions. On the surface, those words might look routine, but following them can make the difference between a safe, effective product and a useless or even dangerous one.
I used to ignore storage advice, thinking a cool kitchen cabinet would work for nearly anything. Over time, I saw firsthand how temperature and humidity can ruin a pricey supplement or turn household cleaners into mystery sludge. Not everyone has the same setup at home, and real life isn’t a laboratory. Still, the reasons behind those guidelines matter.
Products are made with ingredients designed to stay stable in predictable conditions. My experience storing antibiotics for my dog taught me that heat and light break down both effectiveness and safety. According to the World Health Organization, high temperatures speed up chemical reactions in medicines. This means the stuff you rely on during flu season or allergy attacks won’t work as intended, or worse, grows harmful mold and bacteria. The same lesson applies to certain foods—unrefrigerated eggs during a midsummer heat wave become a science experiment more than a breakfast staple.
Moisture creeps up on you. That harmless-looking packet of silica gel in electronics boxes isn’t just extra trash. Moisture damages powders, tablets, snacks, even cleaning supplies. A University of Minnesota study highlights how humidity can cause medicine tablets to crumble or stick together. In one personal example, aspirin tablets in an old bathroom cabinet turned gummy in a month from dampness, which I only noticed on a rushed morning with a headache. By that point, potency was anyone’s guess.
Direct sun destroys pigments and vitamins. I once left a multivitamin jar on a sunny windowsill by mistake; a week later, the colors faded and the smell turned odd. Some chemicals react with UV light faster than others, and pharmaceutical-grade storage recommendations stress keeping things “in a cool, dark place” for a reason. Sun can break down packaging, too—not just what’s inside. So storing anything light-sensitive in opaque containers or closed cupboards makes sense, even if it seems like a hassle.
So much depends on clear labeling—details like “keep refrigerated, do not freeze” aren’t there for show. If a product comes with a specific temperature range, it’s a sign to use a fridge thermometer and avoid overpacked freezers where air doesn’t circulate. In my kitchen, I started dividing items: pantry-safe, cool-cabinet, and fridge-only. For those who live somewhere humid, silica gel packets keep things drier in small spaces and airtight containers go a long way. It helps to check old containers for changes in appearance, smell, or texture since those early signs show before things become unsafe.
Quality always comes back to respect for the way a product is made. Everything from storage at the warehouse to your own home has a part in keeping things safe. Following instructions on the package isn’t busywork—it’s putting trust in the science and evidence behind those products. Using some simple habits based on real experience and strong, research-backed guidelines keeps everyone healthier and budgets under control.
Anyone working with silanes in a real-world lab knows about trial and error. Sometimes, combining new silane molecules feels as risky as making a mystery sauce for a family dinner. (3-Acryloxypropyl)methyldimethoxysilane is no exception. As someone who’s mixed, measured, and sometimes even mopped up silane spills on a concrete floor, I’ve seen what happens when different silanes meet. The science gets lively fast, but it rarely feels tidy.
Each silane features a silicon “anchor” and a group hanging off the other end. The anchor wants to grab water or stick to surfaces; the other group might bring an acrylic handle, an amine hook, or an epoxy hand. (3-Acryloxypropyl)methyldimethoxysilane brings an acrylate, so it helps form crosslinked plastics, adhesives, or coatings that withstand some abuse. The methyldimethoxy part gives it a chance to react with moist surfaces or glass.
Lab folks keep eyeing blends for phase separation. Put (3-Acryloxypropyl)methyldimethoxysilane in with another silane, most times you don’t spot clumping or obvious trouble if their solvents match. It cooperates pretty well with other trialkoxysilanes—like methyltrimethoxysilane, vinyltrimethoxysilane, and most amino-functional silanes. The big thing is hydrolysis rate: some silanes release methanol quicker, others lag behind. Mix a fast one with a slowpoke, and separate layers or weird clumps can develop over time, especially if there's some moisture in the air or on the mixing tools.
The real red flag comes up when acidic or basic silanes meet. Aminosilanes sometimes fight with acrylates, speeding up hydrolysis or crosslinking before you’re ready. Not all lab disasters blow up in your face, but sometimes your batch gets gummy before you can even finish mixing, wasting a whole afternoon’s work.
I’ve watched production lines grind to a halt because two silanes “looked fine” in a datasheet but didn’t cooperate in a mixing tank. That’s expensive. Coating durability or the moisture barrier can change just from a poor match. The right blend builds lasting adhesion to plastic, glass, or metal. The wrong combo turns a high-performance coating into a flaky mess.
Instead of guessing, run a few small test mixes before scaling up. Keep everything dry during blending. Use freshly opened chemicals. If the blend’s for a coating, cure it in stages to spot early failure. Where possible, pair similar hydrolysis rates. Don’t rush the research—watch for changes at every step. Analytical tools like FTIR reveal early crosslinking before it ruins your batch, so pull small samples often.
Manufacturers offer plenty of guidance, but they don’t see your unique lab quirks, humidity, or scale. If you spot unexpected haze or phase separation, consider changing the mixing order or stabilizing with a different solvent system. If nothing else works, shift to a one-pot blend that uses functionalized silanes from a single family. It cuts down surprises and gets more predictable results, time after time.
No mix ever feels truly routine. Take care with every blend, trust small-batch experiments, and listen to what your product tells you in the curing room. Compatibility isn’t about theory—it shows up in the work you hold in your hands at the end of the day.
Working with chemicals doesn’t just ask for attention to detail; it asks for a real respect for what these substances can do. I remember watching a friend burn his hand badly during a high school experiment, all because he figured “it was just a bit of acid.” Hospital trips change perspectives pretty quickly. People rarely get a second chance to take safety seriously after a big mistake, so those warning labels and lengthy safety sheets deserve an honest read.
Let’s get everyday about this: gloves on your hands, goggles over your eyes, lab coat covering your clothes. Simple gear, big protection. Gloves made from nitrile or neoprene give far better protection than old kitchen gloves from under the sink. Safety goggles, not just regular glasses, shield from quick splashes that can blind in seconds. Long sleeves and long pants keep splatters off your skin. Closed-toe shoes, never sandals.
Read the label, every time. Even if someone taught you the routine a hundred times before, chemicals change between suppliers and batches. Each label and safety data sheet spells out the right storage, what to do if you spill, and how to keep air quality safe. Good ventilation isn’t fancy: crack a window wide, set a fan to push fumes away, or work under a hood.
A surprising number of hospital visits follow from chemicals stored in unmarked bottles or within kids’ reach. Keep chemicals in original containers. Label every bottle. Don’t stack bottles in hot sunlight, near heaters, or beside food. Lock up dangerous liquids and return the lid quickly—vapors escape.
Bleach and ammonia belong nowhere near each other; chlorine gas sends people straight to the ER. Never mix chemicals unless a reliable source—like the manufacturer or a peer-reviewed source—clearly spells out that it’s safe. Reactions can turn lethal with one careless pour.
Spills demand calm thinking. If something splashes, leave the area for fresh air. Flush your skin or eyes with running water for a good fifteen minutes. Clear everyone out if strong smells linger or if anyone coughs or feels dizzy. Call local poison control for instructions and keep emergency numbers close by. Every lab or workshop needs an easy-to-find eyewash bottle and a bucket of absorbent material, which absorbs chemical puddles before they move elsewhere.
Wear gear even for “one quick pour.” Cuts, dryness, or sensitive skin get worse with chemical exposure; even fumes can cause headaches, long-term nerve damage, or respiratory issues. Look out for the people working alongside you. If someone seems disoriented or develops a rash or cough, don’t brush it off. Chemicals take a toll over time.
Accidents don’t just cost physical health; they cost time, money, and stress for families and communities. Training sessions, quick reminders, and a workplace culture that rewards speaking up help more than disciplinary rules ever will. Encourage questions. Keep safety instructions visible. The little tasks—closing bottles, washing hands, wiping surfaces—make labs and homes safer for everyone, every day.
Every chemist or production manager who regularly deals with silane coupling agents likely keeps a careful eye on the dates stamped on bottles. (3-Acryloxypropyl)Methyldimethoxysilane doesn’t forgive much mishandling or forgetfulness. Most chemical suppliers set its shelf life at about twelve months after it leaves the warehouse, provided it stays tightly sealed, away from moisture, and in a cool place. That’s not an arbitrary number; even from personal experience working in research teams, once this compound sits exposed to the tiniest bit of water, it begins to turn in the bottle.
Ignoring storage conditions turns this clear liquid yellowish and sticky — a sure sign hydrolysis kicked in. The methoxy groups start breaking off, creating silanols and, pretty soon, leading to unwanted polymerization. That kind of byproduct spells trouble in a research lab or an industrial setting because formulations can lose performance. Back at the bench, I’ve opened bottles older than a year, sometimes discovering a gelatinous mass instead of the pourable reagent we needed. Attempts to salvage those batches never ended well: coatings peeled, adhesives lost grip, and project delays stacked up. It all traced back to that shelf life warning.
The 12-month expiration mark reflects both chemical stability and safety data. Increased acidity or traces of water in the storage area speed up degradation. At ambient lab temperatures — typically around 20–25°C (68–77°F) — unopened bottles make it to the end of their shelf life in usable condition. Once opened, every recapping invites a bit more air and humidity inside, so in practical terms, opened containers tend to last several months less than their unopened twins.
Manufacturers know exactly how temperamental these organosilanes act, which is why some suppliers ship (3-Acryloxypropyl)Methyldimethoxysilane in small bottles or suggest storing the chemical in dry, inert gas (like nitrogen) conditions. The acrylic group remains susceptible to radical formation under UV or heat, further stressing the need for controlled environments.
Burning through batches of expired silane wastes more than money. Cohesion and adhesion fail, leading to faulty products or failed research. Experience — and a few too many ruined experiments — taught me the value of marking opened dates on bottles and strictly following rotation protocols. The frustration of repeating tests due to expired stock leaves a lasting impression. Professional standards demand accuracy, and reliable results only come from uncompromised reagents.
Strict inventory checks and dated labels serve the whole team better than guessing or risking shortcuts. Small batch purchasing minimizes the risk of leftover, unused stock. Keeping bottles in clean, dry cabinets with limited exposure to light and temperature swings protects shelf life, just as manufacturers recommend. Ensuring a desiccator or inert atmosphere for partial bottles pays off over time, especially for teams working on high-value or sensitive projects.
Attention to shelf life might seem tedious, but in my career, close monitoring and adherence to proper storage gave consistent results and safer labs. Companies and researchers alike benefit from understanding not just that (3-Acryloxypropyl)Methyldimethoxysilane has a clear shelf life, but exactly why. Saving on waste, reducing errors, and protecting results depend on careful stewardship from delivery to disposal.
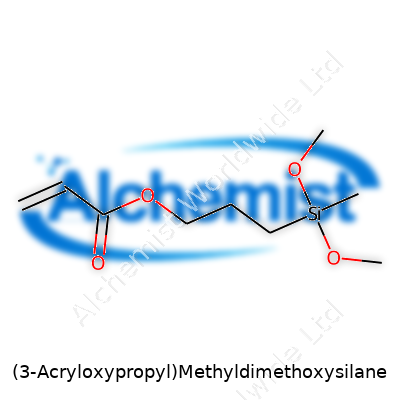
| Names | |
| Preferred IUPAC name | 3-(Propenoyloxy)propyl-dimethoxy-methylsilane |
| Other names |
γ-Methacryloxypropylmethyldimethoxysilane 3-(Methacryloxy)propylmethyldimethoxysilane Silane, (3-methacryloxypropyl)methyldimethoxy- Methyldimethoxy(3-methacryloxypropyl)silane |
| Pronunciation | /θriː-əˈkrɪlɪˌɒksiˌproʊpɪl ˈmɛθɪlˌdaɪˌmɛθɒk.siˌsaɪleɪn/ |
| Identifiers | |
| CAS Number | [65799-47-5] |
| Beilstein Reference | 146687-47-8 |
| ChEBI | CHEBI:132970 |
| ChEMBL | CHEMBL4153722 |
| ChemSpider | 87313265 |
| DrugBank | DB15973 |
| ECHA InfoCard | String: 100.117.825 |
| Gmelin Reference | 1396607 |
| KEGG | C14147 |
| MeSH | D016628 |
| PubChem CID | 15736512 |
| RTECS number | RR1350000 |
| UNII | 1LR19U0N43 |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID9041758 |
| Properties | |
| Chemical formula | C9H18O4Si |
| Molar mass | 234.34 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Characteristic |
| Density | 1.02 g/cm3 |
| Solubility in water | miscible |
| log P | 0.5 |
| Vapor pressure | 0.6 mmHg (20 °C) |
| Acidity (pKa) | 4.07 |
| Basicity (pKb) | pKb = 3.5 |
| Refractive index (nD) | 1.4290 |
| Viscosity | 6 mPa·s |
| Dipole moment | 3.06 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 489.6 J/mol·K |
| Std enthalpy of combustion (ΔcH⦵298) | -2120.7 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS05 |
| Signal word | Danger |
| Hazard statements | H315, H318 |
| Precautionary statements | P280, P261, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-2-2-NA |
| Flash point | > 102°C |
| Lethal dose or concentration | LD50 (Oral, Rat): > 2,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for (3-Acryloxypropyl)Methyldimethoxysilane: Not established |
| REL (Recommended) | 200 - 1000 ppm |
| Related compounds | |
| Related compounds |
(3-Aminopropyl)methyldimethoxysilane (3-Glycidyloxypropyl)methyldimethoxysilane (3-Methacryloxypropyl)methyldimethoxysilane (3-Chloropropyl)methyldimethoxysilane |