
In the early days of silane coupling agents, chemists focused on bridging organic and inorganic surfaces. During the boom of synthetic materials in the second half of the twentieth century, researchers needed something that would make glass or mineral surfaces bond more tightly with advanced resins or polymers. 3-Acryloxypropyltrimethoxysilane showed up as an answer to that demand. This compound did not just slip into the market; it carved out a niche with its unique structure. I recall leafing through old journals where scientists detailed the challenge of getting acrylate groups to work together with silicon — a task that means tackling hydrolytic stability and reactivity at once. The commercial adoption took off in the late 1980s, especially as composite technology in the automotive and electronics industries needed better adhesion and durability.
Every time I open a catalog for specialty chemicals, 3-Acryloxypropyltrimethoxysilane stands out with its versatility. Structurally, it combines a reactive acryloxy group with a trimethoxysilane tail, so manufacturers use it both as a surface modifier and as a monomer for polymer chemistry. This dual nature means it works in coatings, adhesives, sealants, and even advanced composites where durability against moisture matters. The real beauty lies in how it reacts: the acryloxy group copolymerizes with acrylics, while the silane side bonds tightly to glass, mineral fillers, and metals.
If you have ever handled 3-Acryloxypropyltrimethoxysilane in the lab, you might notice its faint, pungent odor, reminiscent of both esters and mild solvents. It comes as a clear, colorless to pale yellow liquid, not particularly viscous. With a molecular weight around 248 g/mol, its boiling point sits at about 100°C under reduced pressure. This chemical readily hydrolyzes in the presence of water, releasing methanol — something you learn quickly if a bottle is accidentally left uncapped on a humid day. Its density at room temperature sits just below 1.1 g/cm³, and it mixes easily with alcohols, esters, and ketones, but reacts with water, which makes handling a bit of a balancing act between efficiency and caution.
Manufacturers usually label 3-Acryloxypropyltrimethoxysilane with purity ranging from 97% upwards, highlighting the need for tight quality control in both packaging and shipping. Labels warn of its reactivity with moisture and its irritant properties. I remember safety briefings where instructors stressed double-sealing bottles and running periodic Karl Fischer titrations to check water content. Typical specifications include refractive index, color (APHA scale), and the exact percentage of the active silane, since trace water or acidic contaminants can start unwanted polymerization during storage. Compliance with REACH and GHS standards shows the industry is serious about labeling transparency and transportation safety.
Chemical producers synthesize 3-Acryloxypropyltrimethoxysilane through an esterification reaction. The process usually starts with 3-hydroxypropyltrimethoxysilane and acrylic acid, using a catalyst like sulfuric acid or p-toluenesulfonic acid. After the heated reaction, water byproduct must be removed, often with azeotropic distillation, to push the reaction toward completion. From my experience in pilot-plant scaleups, handling the acrylic acid's tendency to polymerize and the silane's sensitivity to hydrolysis makes the work demanding. Operators store the crude product under nitrogen and distill under reduced pressure to ensure a high-purity final product. Any deviation in reaction conditions leads to either gelation problems or a drop in yield, so process control cannot be an afterthought.
The unique ester-silane structure of this molecule makes it incredibly reactive while also prone to side reactions. The acryloxy end can copolymerize with other vinyl monomers, forming crosslinked polymers used in paints or adhesives. The methoxysilane group, on the other hand, hydrolyzes to form silanols, which then condense onto surfaces like glass or metal, creating a strong chemical bridge. In practical terms, chemists exploit both reactions in sequence or simultaneously, tuning pH and temperature to get the desired structure on surfaces or within bulk materials. I have seen research groups experiment with this compound as a starting point for grafting other functional groups, expanding its role in hybrid materials and tailor-made surface treatments.
Trade names and synonyms for 3-Acryloxypropyltrimethoxysilane fill entire columns in technical datasheets. You might see it listed as Silquest A-174, KBM-5103, or even by its less catchy systematic name: gamma-methacryloxypropyltrimethoxysilane. Some manufacturers emphasize "gamma" while others opt for "3-," reflecting the location of the functional group. No matter the label, these names refer to the same essential chemistry, which simplifies ordering samples but complicates patent searches.
Every chemist learns early on that handling trimethoxysilane derivatives means strict adherence to lab rules. Breathing in the vapors can irritate your nose and throat, and skin contact leaves a lingering burning sensation. Methanol release during hydrolysis adds more risk — this is not something you want to inhale or let near open flames. Facilities working with this compound install local exhaust and provide chemical splash goggles, gloves, and ventilated storage. Training sessions often focus more on what happens if water leaks into a drum or how to safely dilute and neutralize spills than on routine uses. Safety Data Sheets from major suppliers spell out these requirements — not as legalese, but as practical steps to keep chemical workers healthy.
Industry puts 3-Acryloxypropyltrimethoxysilane to work strengthening the bond between organic polymers and inorganic surfaces. Manufacturers use it in fiberglass composites for automotive panels, winding up with bodywork that resists cracking in harsh weather. Electronics firms incorporate it in encapsulants and dielectric layers, counting on its ability to provide moisture resistance without sacrificing electrical performance. Paint formulators use it as an adhesion promoter, guaranteeing that coatings stick tightly to metal and concrete, surviving cycles of heat, cold, and wetness. Recently, some emerging fields have started trialing it in dental resins or 3D printing materials, showing that its chemistry finds value even where demands on performance only keep rising.
R&D labs, both in universities and private firms, chase the promise of smarter adhesives and longer-lasting paints with 3-Acryloxypropyltrimethoxysilane at their core. Recent publications track how changing the ratio of this compound in a composite alters its thermal and mechanical stability. Across conferences, scientists present results on improved resistance to delamination or cracking in tough field conditions. I’ve seen firsthand how modifying the silane’s hydrolysis kinetics lets you fine-tune the time window for crosslinking during manufacturing. Some research even blends this silane with nanoparticles or other reactive monomers to reach entirely new regimes of heat, UV, or water resistance. The appetite for data and performance metrics here never seems to slow down.
Toxicologists approach 3-Acryloxypropyltrimethoxysilane with both caution and curiosity. Acute exposure studies show irritation at low levels, and longer-term research keeps an eye on potential chronic effects like respiratory sensitization or contact dermatitis. Animal studies have found relatively low toxicity by oral and dermal routes, but the methanol byproduct from hydrolysis brings its own hazards, including central nervous system effects and potential blindness at high exposures. Regulatory bodies in Europe and the US require clear labeling and restrictions for industrial and professional use, and research continues into how to reduce worker exposure or swap in alternatives for especially sensitive applications. These efforts matter — not just at the plant but through the entire supply chain.
The pathway forward for 3-Acryloxypropyltrimethoxysilane looks busy. Demand for strong, waterproof adhesives and high-performance composites shows no signs of slowing. Researchers focus on making its synthesis more sustainable, cutting down on emissions and waste, and refining purification to deliver materials with even fewer impurities. The push toward greener chemistries may lead to new derivatives that offer similar utility with lower health risks or environmental footprints. Emerging sectors like renewable energy, advanced optics, and energy storage already experiment with these silane-modified surfaces, driving technology updates at a steady pace. There’s plenty of work left, from basic reaction development to practical hazard reduction, and each new project sharpens the picture of what 3-Acryloxypropyltrimethoxysilane can do next.
3-Acryloxypropyltrimethoxysilane might sound like something reserved for a scientist’s laboratory, but this compound quietly shapes the world around us. This silane shows up in applications that blend the tough world of materials science with the things we see and use all the time. It doesn’t just sit on a shelf; it acts as a bridge between materials and helps bring strength and durability to products most of us rely on every day.
Modern manufacturing pushes the limits of what materials can do. Composite materials need to stick together under pressure, in harsh weather, or when they get banged up. Here’s where 3-Acryloxypropyltrimethoxysilane steps in. This molecule attaches to both organic surfaces like plastics or resins and inorganic surfaces like glass or metal. It creates chemical bonds between these materials, boosting how well they stay together. That’s especially helpful in fiberglass products, advanced coatings, dental adhesives, and even wind turbine blades. If you’ve ever wondered why fiberglass bumpers or epoxy floors seem so tough, this compound often plays a vital role.
Old paint peels, glues lose their hold, and water finds its way into all sorts of cracks. 3-Acryloxypropyltrimethoxysilane gives paints, varnishes, and adhesives extra muscle. It lets these coatings stick to surfaces like metal or glass in ways that regular paint or glue just can’t manage. That means fewer problems with rust on your car or peeling paint on roadways, and greater weather resistance for infrastructure projects. The buildings and bridges that last longer with fewer repairs owe some of that resilience to the behind-the-scenes chemistry of silane treatments.
Many electronics have plastic parts tightly bonded to glass or metal. For smartphones, this can mean better scratch resistance or less chance a screen will shatter from a minor drop. In the world of fiber optics, 3-Acryloxypropyltrimethoxysilane helps coax cables and lenses to hold together, which helps with the fast, reliable internet many of us now take for granted. My own experience with device repair has shown how much better modern glues hold up compared to those in the early 2000s, and silane compounds are part of the reason why cracked screens are less common or catastrophic today.
The compound doesn’t only matter for large-scale manufacturing. Dentistry uses 3-Acryloxypropyltrimethoxysilane to help fillings and crowns bond with the natural structure of teeth. This helps fillings last longer and stay put despite biting, chewing, or hot and cold foods. For medical devices, keeping all the pieces together can mean the difference between a successful surgery and a failed procedure. The compound’s ability to bond with both metal and ceramic matters here, making equipment stronger and more reliable.
No compound should get a free pass when it comes to safety or environmental impact. Long-term studies point to low toxicity in regular handling, though workplace safety calls for gloves, goggles, and ventilation. Responsible use relies on manufacturers following best practices for both production and disposal, avoiding residue release into water sources. Responsible sourcing and handling tie into trust, and with public access to safety data improving, it’s easier for workers and end users to make informed decisions.
3-Acryloxypropyltrimethoxysilane pushes material science forward, but progress always comes with challenges. Greener chemistry research aims to keep the benefits while reducing potential waste and exposure. Open debate about chemicals in our environment keeps researchers looking for better answers, not just in the lab, but through working with regulators and everyday users. That sort of transparency and commitment to safety lines up with what people expect from the companies behind the products they rely on.
There’s a clear reason scientists keep turning back to 3-Acryloxypropyltrimethoxysilane in coating, adhesive, and composite work. This molecule packs two different powers in one—a reactive acryloxy group and a silane unit. The mix unlocks all kinds of bonding tricks, both with organic and inorganic surfaces. Spend any time in a chemistry lab or on a factory production line and you spot the difference: materials stick together better, and surfaces last longer under stress.
At its core, the molecule holds three methoxy groups connected to silicon and a reactive acrylate tip on the other end. Those methoxy groups bring it close to glass, minerals, and metals, triggering a chemical handshake. The acrylate arm gets involved in polymerization, blending right into acrylic, epoxy, or urethane systems. Once crosslinked in a material matrix, the result is fewer failures at the boundary between layers—and better resistance against peeling or breaking down.
Plenty of research backs up the increased adhesion from treating surfaces with 3-Acryloxypropyltrimethoxysilane. In construction, for example, it boosts the sticking power of sealants applied to concrete or stone. In electronics, it locks coating layers on glass-reinforced circuit boards. By building these siloxane bridges at surfaces, cracking along joints drops dramatically. Factories see fewer callbacks or product damage, saving time and resources down the line.
Anyone who’s painted a fence or sealed a driveway learns fast how much sun, rain, and temperature swings can wear surfaces out. This silane’s chemistry helps out by forming a moisture barrier. Lab tests show lower water uptake and slowed hydrolysis in finished goods compared to untreated or traditional blends. Companies using this chemistry produce parts that better handle humidity swings—think of phone screens or outdoor adhesives that keep performing between winter snow and summer heat.
No one ignores the impact of synthetic chemicals anymore. Like many silanes, 3-Acryloxypropyltrimethoxysilane can cause irritation if misused. Worker safety comes first: protective gloves, eye shields, and solid ventilation keep risks manageable. Waste handling follows local regulations to prevent environmental contamination. As with most industrial silicones, it doesn’t break down easily in nature—minimizing released amounts and recycling coated products where possible becomes smart policy. Industry moves toward greener processes by using only the minimum needed and recovering solvents after use.
The future for this silane looks bright, especially as engineers demand lighter, tougher, and more weatherproof materials in cars, wind turbines, and medical devices. By dialing in exactly how this molecule gets added—be it a primer, a coupling agent, or a crosslinker in resin—creators push the performance envelope. Research continues to reveal new tweaks for lower processing temperatures, better long-term health ratings, and next-generation composites that meet both economic and environmental expectations.
Every chemical in the lab seems like it tries to find its way into a story worth a warning. 3-Acryloxypropyltrimethoxysilane counts among those that take a bit more respect. It’s a colorless to pale yellow liquid that can play a big role in composites, adhesives, and coatings, but that usefulness doesn’t cancel out genuine safety concerns. Those of us who spend time around silane compounds know that a careless moment or a poorly sealed container can snowball into big problems: ruined product, nasty fumes, maybe even a health crisis.
Hazard data reminds us this chemical can irritate eyes and skin and the vapor can put a dent in your lungs or nose. The magic word in its long name, “trimethoxysilane,” tells you that it reacts with water to release methanol—a risky byproduct. In my own lab experience, I’ve seen what happens when trace moisture gets in a bottle—the next opening gives off a sharp stinging smell, and the bottle cap almost always sticks.
People often debate which detail matters more, but with this liquid there are no shortcuts. I’ve made a habit of checking the cap every time, making sure it’s tight and choosing high-density polyethylene or glass bottles with solid, Teflon-lined seals. Keeping the container out of direct sunlight lets it last longer and cuts down on decomposition or pressure build-up. The label should show the date opened and contain basic hazard info.
It’s not about hoop-jumping for paperwork’s sake; it’s about protecting yourself and everyone else who grabs that bottle down the line. Indoor storage should stick to a temperature range a little above freezing but below 30°C—no one wants fire, sticky goo, or a bottle ready to burst. Any fridge or cool storage area you use needs dedicated space for chemicals like this, far from food or drink. Flammable liquid cabinets offer peace of mind in bigger facilities.
You learn early that skin contact with this kind of silane isn’t simple discomfort. The stuff absorbs slowly and invisibly, so gloves matter—nitrile works better than latex. Pouring and measuring calls for a fume hood or at least strong ventilation, especially in older labs where HVAC might not keep up. Eye protection comes before opening the bottle, not after. Disposable lab coats or sleeves can save a lot of laundry and surprise rashes.
The stories usually start with a forgotten drop on a benchtop. Using absorbent pads or secondary containment reduces cleanup drama. If there’s ever a spill, people should reach for spill kits designed for organosilanes, not just everyday wipes. Methanol formation in contact with moisture makes each spill a little more urgent; opening windows or running exhaust fans helps keep vapor below harmful levels while cleaning.
No veteran chemist would hand this chemical to a new assistant without clear directions. Written procedures—placed in plain sight—save headaches. GHS-compatible labels and up-to-date Safety Data Sheets should live right next to the storage cabinet. In workplaces with turnover, reviewing the SDS before each new task keeps everyone sharp.
Safe habits stick because one bad day can lead to hours in the eye-wash station or costly disposal jobs. Labs and workshops grow a culture of care by setting these examples and giving each person room to speak up before, not after, small risks jump the line into emergencies.
Ask anyone who has spent much time in a lab or on a factory floor: chemicals and safety go hand in hand. 3-Acryloxypropyltrimethoxysilane, often found in the world of adhesives, coatings, and treated glass, looks pretty is just another mouthful of a name for something with serious considerations. Workers use it to improve binding between organic and inorganic surfaces. That job comes with risks, and I remember the first time I opened a barrel — the smell raised eyebrows and the label warnings reminded us this isn’t just water with a fancy name.
Open a safety data sheet for this silane, and you’ll notice warnings: skin and eye irritation, respiratory problems if inhaled, potential allergic reactions. Handling this monomer gets worse without gloves, goggles, and good ventilation. I’ve seen coworkers with red, itchy hands, and many only learn the importance of PPE after a bad day mixing resins or coatings. That’s a real issue in small workshops where safety gear gets skipped to save time.
Studies signal that inhalation over time carries the most significant risk, especially with liquid or vapor in poorly ventilated areas. A 2017 review published by the European Chemicals Agency showed silanes like this one can sensitize skin and irritate the lungs at relatively low levels. Chronic exposure remains the big unknown; there is no evidence it causes cancer, but no one wants to be the unintentional test subject, either.
I always try to look at more than just personal safety. Smaller companies often overlook what happened to waste, rinsed beakers, and leftover product. Many silanes break down in the environment, but this doesn’t mean they are totally harmless. Right now, the EPA lists similar compounds as possible water pollutants, and research from the 2020s suggests long-term effects on aquatic life if runoff accumulates. There’s no everyday testing down the drain, so the real impact accumulates in tiny steps, which later become big problems.
The quickest way to cut risk is better education, something I’ve seen work firsthand. New hires learn safety habits from watching veterans, but mistakes happen when companies skip official training. Posters and safety data sheets help only if someone stops to read them. Hands-on lessons with mock spills or burn kits show everyone what happens if protocol gets ignored for even one day. Safety measures like gloves, fume hoods, and eye wash stations go from optional to routine only if people grow used to them.
Shifting from solvent-based to waterborne systems also helps. Modern chemistry gives us better choices, and I’ve worked with shops that reduced accidents by swapping out worse offenders. Managers who foster reporting — making sure even near-misses get logged — keep people alert. On a bigger scale, upstream suppliers should label these materials accurately and push customers toward greener, less hazardous versions where possible.
3-Acryloxypropyltrimethoxysilane doesn’t grab headlines, but that’s exactly why attention matters. The biggest risks come from routine and overconfidence — skipping a glove, rushing through a procedure, ignoring a splash. Respect for these chemicals grows from a culture of shared stories and honest lessons, not just rules on a wall. Community health, clean water, and safe workplaces depend on it.
I remember the first time I worked with 3-Acryloxypropyltrimethoxysilane in an industrial coating project. Folks threw numbers around, but finding a reliable recommendation took effort. Most experienced chemists settled on somewhere between 0.5% and 2.0% of the total resin weight for most coupling or priming applications. Using less than 0.5% usually did not give the improved adhesion we were after, and using more brought its own share of headaches: sticky surfaces, odd film properties, and higher costs with no added benefit.
Silane coupling agents act like a bridge between organic materials and minerals, such as those used in fillers or glass fibers. When people use too little, the “bridge” looks more like a set of stepping stones. Think about trying to cross a creek on too few rocks – you end up wet. Too much, and not only does the bridge soak up the budget, it can leave behind siloxane oligomers or interfere with crosslinking. These side effects break down product reliability and can affect long-term performance, particularly in damp or humid conditions.
Published studies from Dow and Momentive technical teams echo what practitioners say. Using 1% of 3-Acryloxypropyltrimethoxysilane (by resin weight) often strikes the right balance for adhesion and stability in coatings, sealants, and glass fiber sizings. The Dow Handbook on Silane Coupling Agents points out that going above 2% brings diminishing returns and extra risk to film clarity and mechanical properties. In my own work, bumping up to 2% helped bond on tricky surfaces, but beyond that, cure times stretched longer and shelf life started to drop.
People sometimes forget that it’s not just about the amount, but the way the material enters the system. In water-based formulations, we always pre-hydrolyze 3-Acryloxypropyltrimethoxysilane for 30 minutes in clean, pH-adjusted water. Anything too acidic or alkaline quickly turns the silane into useless lumps. In solvent-based products, folks add it directly to the resin, blending at low shear for 10 to 20 minutes. Skipping these steps led us to clumping and poor product quality before.
Treating 3-Acryloxypropyltrimethoxysilane with respect limits personal and environmental risks. Direct contact with eyes or skin stings, and prolonged exposure over months can cause allergic reactions. MSDS sheets from Evonik and similar suppliers highlight nitrile gloves, splash goggles, and adequate ventilation. Following these guidelines didn’t cost much, yet kept our team healthy through many projects.
Folks running pilot lines or making new formulations should start at the lower end—0.5%—and run side-by-side adhesion or durability tests. These trials pinpoint the best amount for distinct systems. Education and clear workplace procedures help avoid both underdosing and wasteful additions. Equipment that meters silane directly into the batch, instead of by hand, offers much tighter control, especially as production scales up. Reliable suppliers also share application guides and technical help—saving time and rework down the road.
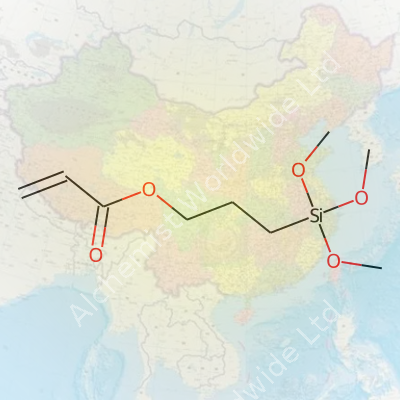
| Names | |
| Preferred IUPAC name | 3-(Prop-2-enoyloxy)propyl trimethoxysilane |
| Other names |
3-(Trimethoxysilyl)propyl acrylate Acrylato-3-propyltrimethoxysilane 3-(Acryloyloxy)propyltrimethoxysilane Acrylsilane A-174 A-174 Silane |
| Pronunciation | /əˌkrɪl.iˌɒk.siˌproʊ.pɪl.trɪˌmɛθ.ɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 4351-37-3 |
| Beilstein Reference | 6512303 |
| ChEBI | CHEBI:132962 |
| ChEMBL | CHEMBL1334850 |
| ChemSpider | 26721 |
| DrugBank | DB14496 |
| ECHA InfoCard | 03f7d36d-33e2-46b8-bad7-6d76a72473b5 |
| EC Number | 219-784-2 |
| Gmelin Reference | 87779 |
| KEGG | C18908 |
| MeSH | D016398 |
| PubChem CID | 66261 |
| RTECS number | AK8225000 |
| UNII | 8I45UVS26O |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C9H18O5Si |
| Molar mass | 248.34 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Characteristic |
| Density | 1.045 g/mL at 25 °C (lit.) |
| Solubility in water | Soluble |
| log P | -0.2 |
| Vapor pressure | <0.1 hPa (20 °C) |
| Acidity (pKa) | 12.98 |
| Basicity (pKb) | 5.7 |
| Magnetic susceptibility (χ) | -6.55e-6 cm³/mol |
| Refractive index (nD) | 1.427 |
| Viscosity | 15 cP |
| Dipole moment | 3.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 324.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -602.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3598.8 kJ/mol |
| Pharmacology | |
| ATC code | No ATC code assigned |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 77 °C |
| Autoignition temperature | 270 °C |
| Lethal dose or concentration | LD50 Oral Rat 8025 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 8,460 mg/kg |
| NIOSH | GY6825000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 50 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
3-Methacryloxypropyltrimethoxysilane 3-Glycidoxypropyltrimethoxysilane Vinyltrimethoxysilane 3-Aminopropyltrimethoxysilane 3-Chloropropyltrimethoxysilane |