
Back in the mid-twentieth century, the demand for advanced silanes kickstarted a wave of innovation. Chemists in Europe and North America dug into new routes for bonding organic compounds with inorganic materials. 3-Aminopropylmethyldiethoxysilane grew out of this era, answering calls from the silane coupling agent industry for stronger adhesives and more stable cross-links. Large-scale adoption followed improvements in purification and distillation, leading to widespread use in glass manufacturing and plastics modification by the 1970s. Early pioneers noted significant improvements in the durability and elasticity of polymer composites, which helped shift market standards.
This silane usually comes as a clear, colorless or pale yellow liquid with a mild amine scent. Suppliers ship it under nitrogen in corrosion-resistant drums and steel containers to prevent hydrolysis. Labs recognize it by codes like A-2110 or silquest Y-11878 and its effective amine number. Many buyers check for compliance with international transport and chemical standards. Producers often list batch analytics to reassure customers about purity and amine content.
3-Aminopropylmethyldiethoxysilane has a boiling point near 96 °C at 10 mm Hg and a density of about 0.88 g/cm³ at 25°C. It has low viscosity, miscibility with common alcohols, esters, and hydrocarbons, and a flash point above room temperature. The ethoxysilyl group resists premature hydrolysis during storage, but kicks off rapid cross-linking in moist air or water. The amine group offers a nucleophilic site that reacts with carboxylic acids and epoxides. The silicon center merges with mineral surfaces—quartz, aluminum, and steel—through robust siloxane bonds.
Every drum carries a lot number, net weight, and manufacturer details. Labels warn about eye, skin, and respiratory irritation. Paperwork details percent purity, hydroxyl content, and moisture by Karl Fischer titration. Data sheets recommend storage below 35°C out of direct sunlight and note compatibility with solvents and typical shelf lives. Experienced users pay close attention to these numbers, as faulty handling reduces yield in surface treatments or coatings.
The most reliable production involves reacting 3-aminopropylmethyldiethoxysilane with trialkoxysilane under carefully dried conditions. Manufacturers add amine and silane precursors in precise ratios into a moisture-free reactor. The batch heats under reflux in the presence of a catalyst (often a Lewis acid), then distills under reduced pressure to yield a pure silane. Silica gel or molecular sieves scrub out residual water. The process demands scrupulous exclusion of moisture, as even trace amounts reduce product integrity.
Functionalizing surfaces with this silane anchors organic layers on metals, glass, and ceramics. The silane’s ethoxy groups hydrolyze into silanols, then condense with hydroxyls on substrates. Reactions with epoxy, carboxylic, or isocyanate chemistry expand its reach. Researchers introduce this silane to amine-capped polyurethanes for building block flexibility. It also forms hybrid organosilicon networks for electronics, where electrical insulation and mechanical stability depend on cross-linking density.
Companies market it under various monikers: “Amino silane,” “3-(Aminopropyl)methyldiethoxysilane,” “Dynasylan AMEO,” “Silquest Y-11878,” and “Z-6011.” The chemical community and regulatory agencies recognize all these terms, yet most labs prefer the simplest description on internal logs. This abundance of synonyms sometimes causes confusion until teams standardize procurement across global locations.
Handling brings some real risks—direct contact irritates skin and eyes, and vapors can cause respiratory symptoms in confined spaces. Proper personal protective equipment includes goggles, gloves, and respirators. Good exhaust ventilation in work zones limits fume accumulation. Most large users require annual safety audits and regular refitting of storage vessels. Spill control plans emphasize inert absorbents and containment dams, as hydrolysis releases flammable ethanol. Training materials often use case studies of past incidents to reinforce lessons, not just tick off regulatory boxes.
Glass fiber industrialists introduced this silane into composites to toughen automotive and aerospace parts. Electronics makers rely on it for adhesion of sealants and encapsulants on circuit boards. It pops up in adhesives, paint primers, and corrosion inhibitors, extending component life under harsh conditions. Paint chemists seek its amine functionality for pigment binding, while formulators in medical devices appreciate its biocompatibility for silicone rubbers. Concrete and mortar scientists blend it in to enrich strength and moisture resistance.
Chemists have chased eco-friendlier versions over the past decade, substituting green solvents and reducing byproducts. Ongoing trials target selective functionalization—adding more tailored substituents for precision tasks. Some labs try anchoring antimicrobial agents using the amine handle, extending coatings’ functional lifespans. R&D centers in Asia and Europe track performance across temperature extremes for electric vehicle and 5G infrastructure. New patents have surfaced around modified silane derivatives for biomedical sensors, looking to harness both flexibility and bioactivity.
Acute studies in rodents show mid-level toxicity, with thinner margins for error than with many hydrocarbon-based additives. Repeated inhalation exposure increases lung irritation. Wastewater containing unreacted silane needs biological or advanced oxidation treatment, since breakdown can yield problematic amines. Industry reviewers increasingly focus on minimizing worker exposure and controlling emissions, driven by the tightening grip of regional chemical regulations. Firms engaged in large-scale production push for closed-loop recycling and solvent reclamation to cut environmental burdens.
The shift into biocompatible coatings, flexible electronics, and greener construction opens new doors for this silane. Investment in direct-to-consumer electronics and medical wearables grows the need for stable, low-toxicity interface agents. The industry faces pressure to swap out harsh catalysts for enzyme-based or aqueous processes. As engineered materials continue to proliferate, reliable coupling agents like 3-aminopropylmethyldiethoxysilane will drive value well beyond yesterday’s commodity resins. Partnerships among researchers, regulators, and manufacturers look set to keep shaping both safety standards and global supply chains for years to come.
Let’s talk about a substance you probably haven’t seen splashed across news headlines: 3-Aminopropylmethyldiethoxysilane. In my work with materials and coatings, this compound shows up more often than many expect. It’s a mouthful, but its role is simple: bridging the gap between organic and inorganic materials, helping surfaces bond in ways plain glues can’t touch.
I’ve seen chemists regard it almost like a trusted tool in their kit. What makes it click is its silane part, which hooks to surfaces like glass, metal, minerals, and even some plastics. The other end—the amine group—latches onto organic molecules including adhesives, paints, resins, and rubbers. Most regular folks never think about why two things—like plastic and glass—sometimes stick together seamlessly. That’s where this compound becomes essential.
Electronics manufacturers count on 3-Aminopropylmethyldiethoxysilane to boost adhesion when mounting chips to circuit boards. I remember visiting a plant where engineers adjusted the silane treatment on the conveyor line, just to ensure the chips stayed on tight through stress, heat, and shaking. Just a tiny amount makes the difference between a reliable device and one that fizzles out on the shelf.
It pops up in coatings and adhesives too. Take paint for glass or ceramics: most water-based paints would flake right off. Add some of this silane to the mix, and suddenly the layer holds up to scrubbing, sunlight, and time. That makes it easier to work with tough surfaces around the home or in factories—no special primers needed. Repair adhesives depend on it, keeping windshields and windows fixed in place after a crash or a storm. Construction teams use it in concrete sealants, storing up more water resistance and less cracking down the road.
Every chemical with the power to bond surfaces also brings risks. I’ve worn gloves and respirators while handling this silane in labs because even in ventilated spaces, vapors can irritate the skin or lungs. Improper handling can lead to headaches or reactions. Regulations require careful labeling and storage, so folks working on industrial floors know how to stay safe. When new staff get trained, it’s the sort of lesson you hope isn’t learned by a trip to the nurse.
Demand for greener chemistry pushes manufacturers to scrutinize compounds like 3-Aminopropylmethyldiethoxysilane. Biobased alternatives and improved process engineering cut down on waste and emissions. Some research labs now develop silanes derived from renewable sources or step up recycling efforts to recover spent silanes. It’s not an overnight fix, but working in sustainability means paying attention to any ingredient along the supply chain that could hurt people or pollute water.
In my experience, the best progress comes through honest talk between chemists, engineers, workers, and environmental experts. No single compound solves all material challenges or environmental concerns, but learning to handle and improve them matters for everyone from gadget designers to home DIYers.
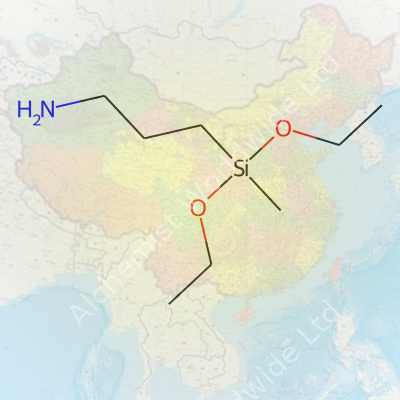
3-Aminopropylmethyldiethoxysilane comes off as quite a mouthful, but the core idea stands simple once unraveled. Its chemical formula is C10H25NO2Si. This tells us everything you need to know about its makeup—ten carbon atoms, twenty-five hydrogens, one nitrogen, two oxygens, and a silicon atom linking everything together.
This silane compound doesn’t just rest on its shelf in a laboratory. From personal experience visiting coatings manufacturers and adhesives plants, I’ve seen it play a quiet but central role. Workers view it as a solid option for linking organic and inorganic materials together. For example, it helps stick glass fibers to resins, making stronger and more durable composites, found everywhere from airplane parts to wind turbine blades.
Silicon-based compounds like this one offer more than raw strength. The amine group at the end of its structure brings a special ability to form bonds with a variety of substrates—metals, ceramics, and even textiles. That's a big deal in industries looking for tougher, longer-lasting products. The diethoxy side-groups help the molecule react with moisture and other reactive sites, anchoring it tightly where other chemicals might only coat the surface.
Every material tells its own story, and not always a smooth-sailing one. For folks working with 3-Aminopropylmethyldiethoxysilane, the main hitch comes from its reactivity. It reacts with water from the air and can start gumming up unless stored properly. I’ve seen production teams frustrated when drums kept in humid environments form gels instead of staying liquid. This wastes materials and slows down operations.
Proper storage—airtight containers and controlled environments—helps stave off these issues. Training workers about these precautions, from plant floor to logistics, makes all the difference. Manufacturers investing in moisture-proof packaging and better facility climate control tend to lose less product and see fewer delays.
Safety teams never gloss over the risk of chemical exposure, and with good reason. The amine part of this molecule can irritate skin and eyes. Long periods without gloves or goggles lead to health complaints. I recall workplace safety reviews where clear labeling, personal protective equipment, and thorough staff training went a long way to lower accident rates. The company not only avoided regulatory trouble but also gained a reputation as a safe employer.
The growing demand for lightweight materials, better adhesion, and more durable coatings keeps compounds like 3-Aminopropylmethyldiethoxysilane in the spotlight. Material scientists keep tweaking production processes for cleaner, greener, and safer outputs. From my talks with researchers, the vision is clear: keep finding methods to reduce environmental impact, cut down on waste, and improve worker safety. Steady improvement, not just in formulas, but in the way people approach the entire supply chain, marks the way forward.
Anyone working with chemicals like 3-Aminopropylmethyldiethoxysilane knows a bottle left on the wrong shelf rarely stays out of trouble. Proper storage comes down to understanding what you’re handling and the mess that can follow poor habits. This compound, with a long name and plenty of uses in adhesives and coatings, brings risks you don’t want left unchecked on a lab bench or in a warehouse.
Heat, light, and air never do chemicals many favors. 3-Aminopropylmethyldiethoxysilane reacts with moisture in the air. That means leaving a cap loose, or letting containers rest near a window, just invites trouble. In my experience, forgetting once about these factors means coming back to an unusable or even hazardous mess. Factories and labs that have seen container seals sticking or labels peeling can tell you how costly that gets.
Storage for this compound calls for a few basic, no-nonsense steps. Place the container in a cabinet that stays below 25°C (77°F). A cool storeroom with little sunlight keeps the product stable. Humidity rolls in uninvited, so always keep containers tightly sealed after use. I once saw someone top up a container and leave the cap just half-twisted, which quickly led to residue forming and the product going bad by the week’s end.
The need for dry, well-ventilated storage goes beyond protecting the shelf life. Exposure to open air starts a chemical reaction that forms byproducts no one wants. That’s not just a waste of money—it can also increase risks of inhalation or skin exposure later on. A dry environment helps ensure that workers handle only what they expected, not an accidental byproduct forming in the drum.
Sturdy, original containers make a difference too. I Don’t reuse old bottles or switch containers unless you’re absolutely sure of their chemical compatibility. Cross-contamination or weak seals open doors to leaks, which quickly snowball into bigger safety issues.
Accurate labeling of all containers, including hazard pictograms and the date received, gives people the information they need before they twist open a cap. I’ve seen too many labs with mystery bottles, and the resulting confusion can make an already risky substance even more dangerous. Labels that fade or fall off breed mistakes—fresh, legible labels help keep things straight and meet the expectations set by safety standards.
Best storage habits go hand in hand with preparation for accidents. Spill kits and safety showers need to be within easy reach. Workers benefit from regular training sessions—not dry lectures, but real walkthroughs—on what to do when dealing with leaks or splashes. I’ve watched teams handle minor spills with confidence only after drills and clear plans were in place. Prepared teams cut down on injuries and keep losses manageable.
Regular inventory checks bring insight into storage conditions. Outdated or compromised stock finds its way to proper disposal, and storage areas stay free of clutter. Monitoring environmental conditions pays off in fewer surprises, longer product life, and, most importantly, a safer workplace. In the end, responsible storage reflects everyone’s commitment to protecting people and protecting the value of what’s inside every drum or bottle.
Anybody who’s spent time in a lab or on a production floor knows that chemicals like 3-Aminopropylmethyldiethoxysilane don’t forgive careless mistakes. When I handled organosilanes my first year in industry, I watched colleagues take shortcuts. More than once, they paid for it with coughing fits, skin rashes, and scrambled cleanup duties. Nobody enjoys those wastes of time—or the paperwork. Safety sinks in once you’ve had to decontaminate glassware because you thought gloves were optional.
This compound isn’t the friendliest to lungs and skin. Its vapors sting the eyes and burn the nose. OSHA lists aminopropyl silanes as potential irritants; research shows they can sensitize skin over time. I ended up swapping my usual latex gloves for nitrile types, after learning latex couldn’t hold up. Sticky, slow-drying residues ruined a few sleeves before we switched to long-sleeve lab coats. Eye protection proved its worth more than once. Lab goggles kept splashes out, especially when cleaning up spills with solvents—some of which seemed to make the stuff spread even faster.
Good airflow transformed how easy it felt to work with this silane. In rooms where the vents actually worked, I noticed far less odor, coughing, and dizziness. Older labs with cheap ductwork trapped vapor pockets close to benches, making headaches far too common. Opening a fume hood pulled vapors away in seconds. I’ve seen coworkers forget this step, then wind up with burning throats and the urge to get outside for fresh air. Even if a process feels “routine,” a switch flipped while pouring, or a leak from a poorly-sealed bottle, will put harsh chemicals into the room. Keep lids on bottles and work under the hood, every time.
Early on, I made the mistake of storing silane bottles near the acids. Bad call—moisture and acid vapors can trigger unwanted reactions, even just from shared air space. Chemical segregation seemed picky, but I found out it stops cross-contamination and valve corrosion. We started labeling every bottle, sealing caps tightly, and putting containers in secondary trays. This helps catch drips, which reduce headaches during inventory checks. Fresh storage keeps the stuff reliable for longer, as some silanes break down when they soak up water from humid air.
Colleagues new to the job often skipped reading the Safety Data Sheet. That habit never lasted long. I learned more from quick toolbox talks and honest stories than re-reading regulations on paper. Peer coaching and clear signage near the bench built better habits. Training sessions that walked everyone through spill kits and waste containers made the process predictable. When emergencies hit, preparation kept panic away.
Every lab should have a working fume hood, PPE at arm’s length, clear labels, and easy disposal steps. Checking protocols and taking five minutes for a safety recap make handling 3-Aminopropylmethyldiethoxysilane routine—without risking health or stopping the workflow. Chemical work never rewards carelessness, but it does respect those who pay attention, share experience, and use the right gear at the right times.
3-Aminopropylmethyldiethoxysilane comes up a lot in labs and manufacturing spaces. Like most organosilanes, it comes with an expiration date. This isn’t just fine print on a label. The shelf life ties directly to keeping products safe, reliable, and cost-effective. Degraded chemicals do real harm—picture a failed adhesion between a surface and a coating, or a reaction that never really gets going.
An unopened container, kept in the right environment, usually stays in good shape for 12 to 24 months. That shelf life depends heavily on temperature and humidity. Direct sunlight, high heat, and moisture shorten it dramatically. Companies who move through inventory quickly tend to have fewer problems. Small labs and workshops stretching a bulk purchase over years see more headaches from breakdowns and contamination.
I worked a stint in coatings development. We stored a lot of specialty silanes just like this one. After a year, the opened bottles would start to show changes—the smell got stronger, a little vinegar-like, and sometimes haze formed inside the cap. Use the chemical at that point and you risk ruining entire batches. One time, a rushed production run picked up a bottle from two years earlier. The finished parts peeled within weeks in the customer’s hands. Repairs ate into profits by the thousands.
Aminopropyl-type silanes contain groups that react with moisture. If any water sneaks into the container, even just from opening the lid, hydrolysis starts up. The silane breaks down and turns into silanols and other byproducts. These don’t bond well when used in adhesives or surface treatments. At best, you get weaker performance. At worst, finished products fail in the field.
Chemical breakdown tends to show itself through color changes. Fresh 3-Aminopropylmethyldiethoxysilane should look clear to pale yellow. If it turns dark or cloudy, that’s a clue to toss it out. Odd smells like ammonia or acetic acid mean trouble, too. You might spot dried residue on threads or caps—another sign water’s gotten in. Testing a small amount before committing a bigger batch can save a lot of money.
Keeping this chemical usable as long as possible comes down to the basics—cold, dry storage and a tight seal each time you close the container. Think about dividing large quantities into smaller bottles, so exposure stays low. Silica gel desiccant pouches inside packages help by sucking up stray moisture. Label every bottle with the date opened. In busy environments, it’s easy to lose track and go months past the expiration window.
Major suppliers compete not only on price, but on clear storage and handling guidelines. They publish safety sheets with precise recommendations: 2 years in cool conditions, out of sunlight, below 25°C, bottles tightly closed. In my own work, following those instructions meant I could trust every batch. Whenever possible, rotating stock so older bottles get used first makes a real difference for bottom lines and quality.
Overestimating the shelf life leads straight to waste and product failure. With chemicals like 3-Aminopropylmethyldiethoxysilane, a little planning and respect for expiration dates avoid huge costs down the line. Even experienced chemists make mistakes, but placing a priority on smart storage rewards everyone involved—from the lab bench to the end customer.
| Names | |
| Preferred IUPAC name | 3-(Diethoxy(methyl)silyl)propan-1-amine |
| Other names |
3-(Dimethylethoxysilyl)propylamine N-(3-(Triethoxysilyl)propyl)amine APDMES Aminopropylmethyldiethoxysilane 3-Aminopropylmethyldiethoxysilane |
| Pronunciation | /ˈθriː-əˌmiːnoʊˌproʊˌpɪlˌmɛθˌɪlˌdaɪˌɪˈθɒksiˌsaɪˌleɪn/ |
| Identifiers | |
| CAS Number | 3069-29-2 |
| Beilstein Reference | 1465065 |
| ChEBI | CHEBI:87573 |
| ChEMBL | CHEMBL2172060 |
| ChemSpider | 2847561 |
| DrugBank | DB08242 |
| ECHA InfoCard | 03a1d1a1-b7f7-4d84-a4e9-e7c6d593d17e |
| EC Number | 245-770-2 |
| Gmelin Reference | 104149 |
| KEGG | C19809 |
| MeSH | D017197 |
| PubChem CID | 122347 |
| RTECS number | UF9357000 |
| UNII | WN4RV7GJ16 |
| UN number | UN3334 |
| CompTox Dashboard (EPA) | DTXSID7020172 |
| Properties | |
| Chemical formula | C9H23NO2Si |
| Molar mass | 219.38 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Aminelike |
| Density | 0.893 g/mL at 25 °C |
| Solubility in water | Soluble in water |
| log P | 0.2 |
| Vapor pressure | 0.6 hPa (20 °C) |
| Acidity (pKa) | 10.5 |
| Basicity (pKb) | 5.84 |
| Refractive index (nD) | nD 1.415 |
| Viscosity | 8-12 cP (25°C) |
| Dipole moment | 4.24 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -400.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -7571.7 kJ/mol |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS07,GHS05 |
| Signal word | Warning |
| Hazard statements | H226, H302, H314 |
| Precautionary statements | P261, P280, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 71 °C |
| Autoignition temperature | autoignition temperature: 245 °C (473 °F) |
| Lethal dose or concentration | LD50 Oral Rat 2413 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 2,400 mg/kg |
| NIOSH | VV9275000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 1 ppm |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
3-Aminopropyltriethoxysilane 3-Aminopropyltrimethoxysilane N-(2-Aminoethyl)-3-aminopropyltriethoxysilane Methyldiethoxysilane 3-Glycidoxypropylmethyldiethoxysilane |