
Chemistry has always been filled with the push to build, tweak, and find new functions for molecules once thought to only exist on paper. In the 20th century, scientists explored how organosilicon compounds could stretch the boundaries of both lab research and industry. 3-Chloropropyldichloromethylsilane landed on the scene as the chemical industry matured, gaining traction because it could bridge the gap between carbon and silicon chemistry. At first, research centered on finding new functional groups for use in polymer development, then curiosity led chemists to explore more specialized applications. As demand for durability in construction, electronics, and coatings climbed, so did investigations into this compound’s synthesis and safe operation. I remember seeing a wave of patents and research papers pop up in the 1970s and 1980s, a clear signal that manufacturers saw real promise behind this molecule. The backbone for 3-Chloropropyldichloromethylsilane’s success came from a drive to make better sealants and smarter, more weather-resistant materials.
3-Chloropropyldichloromethylsilane belongs to the family of organosilicon chlorides. As a specialty chemical, it finds its way into the toolbox of polymer scientists and industrial engineers alike. The compound’s use spans from serving as a precursor for modified silicone resins to facilitating the introduction of functional chloropropyl groups into larger molecules. My work in polymer modification brought me into regular contact with compounds structurally similar to this one—after seeing how a single functional group can change a material’s properties, it becomes obvious why this compound continues to draw scientific interest. Engineers often value it for the stability and reactivity that both the dichloro and chloropropyl groups confer upon downstream polymers, allowing them to tailor surfaces, join unlike materials, or create water-repellent finished products.
3-Chloropropyldichloromethylsilane comes off as a clear or slightly yellow liquid with a pungent odor that is hard to mistake. It weighs about 1.2 grams per cubic centimeter and boils at just over 180 degrees Celsius. People sometimes overlook how volatility and density interact with practical handling, but a heavier vapor tends to settle near the ground, raising both storage and ventilation concerns. The molecule is reactive with water and moisture, which means it will hydrolyze and form hydrochloric acid along with insoluble siloxanes. Its chemical backbone—anchored by both the trichloromethylsilane and chloropropyl moieties—grants an unusual blend of reactivity toward nucleophiles and stability under neutral, dry conditions. Handling any dichlorosilane never feels routine because a small leak can go from nuisance to hazard with just an errant drop of water.
Technical data sheets often present purity levels above 98 percent, but trace impurities vary depending on production runs and supplier diligence. Laboratory bottles usually have clear hazard pictograms and wear the “flammable liquid” and “corrosive” tags under GHS or comparable standards. Suppliers often cite specific gravity, refractive index, vapor pressure, and contaminant thresholds. For storage, guidelines call for dry, inert atmospheres, clearly labeled Hazard Codes (like UN 2987), and specialized seals to guard against gradual moisture ingress. Clear and honest labeling forms the backbone of responsible distribution, sparing buyers the risks associated with ambiguity.
The typical lab synthesis runs a methyltrichlorosilane through an alkylation, where the introduction of a chloropropyl group takes careful stoichiometric balance. Industrial setups favor the direct reaction of methyltrichlorosilane with excess 1,3-dichloropropane in the presence of a Lewis acid catalyst or a copper promoter at elevated temperatures. Controlling by-product formation and ensuring selective alkylation demand rigorous process control—more than one chemist has learned the hard way that impurity buildup can crash a purification step. The world of chemical manufacturing rewards consistency, and the proper wash cycles, temperature profiles, and distillation methods turn what could be a messy mixture into something reliable and commercially viable.
The reactive Si-Cl bonds open the door to nucleophilic substitution, while the chloropropyl group itself can undergo further transformation to offer amines, azides, or alcohols through substitution or reduction. Silanol formation after controlled hydrolysis allows the tailoring of surface functionalities on glass or ceramics—an essential step in making advanced composites and adhesives. Once I watched a batch of silane-coupled glass fibers change a whole production run for a local electronics company, purely because the right surface treatment led to stronger, more reliable jointing in their circuit assemblies. Modification often reflects economic pressure: downstream industries want strength and weather-resistance without adding weight or cost, and compounds like this one deliver that bridge between affordable chemistry and smart design.
Depending on which catalog you open, the compound may show up as dichloro(methyl)(3-chloropropyl)silane, 3-chloropropylmethyldichlorosilane, or with labeling codes unique to each manufacturer. Some suppliers stick with trade names that roll off the tongue to better sell to specialty plastics and elastomer labs. I still see old-timers use the more descriptive IUPAC names, mostly for clarity in technical papers or safety data sheets. Regardless of branding, what matters to users is the clarity of the label and whether supporting documentation lines up with what the literature or regulatory agencies expect.
Working with this compound leaves no room for carelessness; splash goggles, gloves, face shields, and heavy airflow matter. Hydrolysis generates hydrogen chloride gas, so the right fume hood can be the best investment in preventing chronic workplace exposure. Safe transfer protocols limit contamination and spill risks, with double-sealed storage drums kept away from damp spaces. Regulatory requirements keep a close eye on exposure limits—OSHA, REACH, and other agencies periodically review allowed concentrations and mandate thorough training. No one wants to deal with a chemical burn, but the real gamble is longer-term respiratory or mucous membrane damage from repeated low-level exposure.
Polymer production leads the pack, but the story expands quickly. Electronic device manufacturing, adhesives, sealants, construction materials, and high-performance coatings benefit from the chemical’s unique ability to create cross-linked bonds one day and support surface treatments the next. Water-repellent coatings on glass panels for architecture and anti-corrosion layers in marine settings owe something to organochlorosilanes. My first foray into specialty coatings illustrated how a small tweak—switching to a silicon-based precursor—could push durability far beyond the reach of conventional organic molecules. Research labs like the compound for attaching “clickable” handles to surfaces that resist mechanical and chemical aging, a feature increasingly important for wearables and flexible electronics.
Current R&D trends push the envelope for greener, less hazardous production schemes and for applications in nanocomposites. Teams look at ways to adjust the molecular scaffolding for even higher compatibility with biodegradable polymers, laser engraving, or responsive coatings that shift property with a change in environment. The demand for process intensification—making more product with less energy or solvent—keeps chemists rethinking classic approaches. I’ve noticed more collaboration between academic materials scientists and large-scale producers, as both sides chase the goals of circular economies and sharper performance targets.
Toxicological data paints the compound as acutely toxic by inhalation, skin absorption, or if swallowed. Animal studies point to local irritation, damaging effects on internal tissues, and heightened risks from chronic exposure that can linger long after a spill goes unnoticed. Emergency rooms sometimes see cases related to improper handling, and most chemical companies lean heavily on regular health monitoring and air quality testing. Safety data underscores strict personal protection, with clear recommendations on decontamination procedures and medical intervention. Any research project considering this molecule for consumer-facing use always brings toxicologists into the picture from early design stages, aiming to shrink the environmental and health footprint.
The road ahead appears flush with opportunities tied to high-performance materials, moisture-resistant coatings, and the swelling market for “smart” construction products. Growing regulatory scrutiny over volatile chlorinated organics pressures suppliers to shift toward safer packaging and lower-emission synthesis routes. Digital tracking, batch-level traceability, and smarter monitoring tech change how production teams work with chemicals like this every day. Interest also grows in hybrid materials that marry silicon chemistry to green, bio-based backbones, opening the door for a new generation of sustainability-aware products. The biggest difference I see today comes from a sharper focus on lifecycle thinking—researchers measure impact not just in superior performance, but in how long a material resists breakdown, how safely it can be recycled, and whether future generations will inherit safer factories and cities.
Walking down a city street or sitting inside a car, you might not realize how much chemical engineering has shaped those surroundings. Among the long list of industrial chemicals, some have jobs that rarely get public attention. Take 3-Chloropropyldichloromethylsilane. Chemists and manufacturers know it as a go-to compound for making a certain kind of silicone. It does not have a catchy nickname, yet it plays a role in making the products all around us tougher, more reliable, and sometimes downright safer.
3-Chloropropyldichloromethylsilane acts almost like a bridge. Its structure brings organic and inorganic elements together. Once it reaches a factory, workers often use it as a building block for producing siloxane polymers and silane coupling agents. In easy terms, it helps different ingredients bond tightly, which means products like sealants, adhesives, and specialized coatings can stand up to tough environments. These are everyday items in construction, car manufacturing, and electronics.
Take adhesives, for example. Ordinary glue might lose stickiness with moisture, temperature swings, or UV rays. Products built with silicone from chemicals like this one hold strong in spots where cheaper glues would fail. In my own workshop, I have watched cheap caulk peel off a bathroom fixture, while a silicone-based one with a silane backbone keeps water out for years.
Electronics need materials that insulate but remain flexible. The same goes for weatherproof coatings on buildings. Using 3-Chloropropyldichloromethylsilane in the recipe helps manufacturers achieve those traits. I have seen silicone sealants that outlast the metal or glass they protect. That reliability traces back to these sturdy chemical bonds, giving architects and engineers something they can count on for the long haul.
There’s also a subtle side to its usefulness. Silane coupling agents built from this molecule make surfaces easier to bond in the first place. So, factories can join rubber to metal, or glass to plastic, keeping production lines moving smoothly. The automotive sector runs smoother because of it; electronics assembly gets less waste and stronger products.
Chemicals like this one deserve respect. Its high reactivity means it doesn’t belong outside controlled environments. Exposure can put air and water at risk, so companies put strong safety measures in place. Air filtration, proper waste handling, and worker protection make a difference. If ignored, spills or leaks could do real harm, and regulators want careful monitoring for this reason.
The push for green chemistry challenges manufacturers to keep these high-tech benefits while reducing risks. Researchers explore ways to handle and dispose of compounds like 3-Chloropropyldichloromethylsilane without long-term side effects. As industries figure out safer substitutes or better filtration, public health and the environment stand to gain. Responsible use lets us keep the strengths of silicone-based technologies in our daily lives—without rolling the dice on safety.
3-Chloropropyldichloromethylsilane rarely makes headlines, but anyone who works with specialty chemicals knows how unforgiving and hazardous some of these substances can be. I’ve spent time in research labs where mishandling dangerous silanes created emergencies no one wants to repeat. This compound combines volatility, reactivity, and the ability to hit the user with burns, lung damage, and more. Carelessness doesn’t just mean a mild inconvenience; it can put lives on the line.
It carries a nasty punch. A drop on bare skin may burn, even through gloves that can’t handle silanes. Once, a colleague underestimated splash risk and ended up spending hours with Occupational Health after a brief encounter. Eye splashes threaten lasting vision loss. An unfiltered breath risks lung injury, persistent cough, and even long-term damage. These stories aren’t rare; they form the background noise in chemical safety circles.
Open benches don’t cut it for this one. Every handling always belongs in a fume hood or under a glovebox. Fume hoods suck away vapors before they ever reach your face. The inside stays negative pressure, stopping leaks from flowing into breathing zones. Good labs never cut corners on vent checks and sash heights. Keep the sash low—the air moves better. I’ve seen a smoke test convince even seasoned researchers to respect it.
I treat PPE as less of a chore and more like a habit that keeps me working another day. Gloves need to resist both organic solvents and strong acids, so nitrile alone won’t always cut it. Double-gloving with a heavier barrier makes sense here. Face shields and goggles go on together—splashing disciplines nobody. Lab coats ought to have tight cuffs. If there’s a risk of a bigger spill or the project gets messy, chemical aprons and arm guards come out.
Training separates safe teams from disaster-prone ones. Everyone should know the chemical's specific dangers and rehearse what happens if something spills or goes airborne. Keeping neutralizing agents in arm’s reach, like sodium bicarbonate or lime, stops a routine slip from spiraling. Emergency eyewash units and safety showers must work and stay unblocked. I always check my escape path before uncapping a silane. Every drill feels useless—until the real thing happens.
It makes no sense to keep this stuff in glass jars at eye level or beside incompatible chemicals. Dedicated, vented chemical storage—ideally, a flammables cabinet—limits accidents and keeps reactivity in check. Labels aren’t optional, and date everything so old, potentially degraded stock gets tossed before it can turn dangerous. Waste gets segregated, never down the drain. Professional disposal isn't just about rules—local waterways and air stay safer that way.
Via personal trial and too many stories shared among peers, treating 3-chloropropyldichloromethylsilane carelessly just isn’t an option. Hands-on practice with proper tools—syringes for transfer, secondary containment, and proper dilution in hood—makes daily work less stressful. Not everyone learns the hard way if experienced workers pass down what matters: you don’t get a second chance to dodge a bad chemical burn or lung exposure. Real safety grows from experience, but facts and vigilance come first.
3-Chloropropyldichloromethylsilane isn’t the friendliest liquid in any lab. In my own chemistry days, I learned pretty fast that ignoring basic cautions with reactive chemicals like this one leads to cleanup headaches at best, and genuine disaster at worst. What separates 3-Chloropropyldichloromethylsilane from more familiar reagents is its hunger for moisture; the chemical reacts with water, making hydrogen chloride gas and silanols, both of which can burn your skin, eyes, and lungs in a flash.
This experience taught me one clear truth: you store this compound cool, dry, and sealed. Letting it get warm increases its volatility. A temperature-controlled cabinet, tucked away from direct sunlight and heat, offers the peace of mind every tech or chemist deserves. Good air flow becomes crucial, too. Even the best-sealed bottles can leak vapors over time, and inhaling the fumes feels like a punch to the face. I still remember stories from friends working industrial settings—one left a cap slightly loose, and the sharp, acrid smell hit the nose even outside the room. That’s why every spot storing this liquid needs working ventilation, ideally with local exhaust over storage locations.
Glass and high-grade polyethylene containers, with reliably fitted lids, are the real standard for 3-Chloropropyldichloromethylsilane. Metal containers bring in corrosion risks. From my experience, the temptation to improvise with “almost right” bottles leads straight to trouble. Leaky lids or fragile seals? That means acid vapors escaping and attacking both people and nearby equipment. Industrial supply catalogs list containers specifically rated for strong acids and water-sensitive materials. Don’t scrimp here—an extra dollar or two on the right vessel protects far more than just the chemical.
Sloppy labeling turns minor incidents major. The time saved skipping this step evaporates the moment confusion hits. Identify every container with both the chemical’s name and hazard classes. Keep it away from water sources, strong bases, and oxidizers. Mixing mistakes can lead to violent reactions. In one incident I heard about, a mislabeled bottle mixed with a cleaning solution nearly triggered an evacuation thanks to a sudden hydrogen chloride release. Careful records and clear segregation prevent chaos.
Storing chemicals safely means gearing up. I’ve never regretted closing a bottle gloved and goggled up. Rubber gloves, goggles, long-sleeved lab coats—they’re all non-negotiables when handling or checking on this compound. Have neutralizing agents and absorbent pads stashed nearby. Spills with this chemical do not just mean mopping; they demand careful neutralization, plenty of fresh air, and secure disposal procedures. OSHA recommends facilities keep eyewash stations and emergency showers in any room where spills can happen.
Safe storage habits go beyond locked doors and fancy labeling. A safe team keeps each other honest, running drills, updating old storage rules, and sharing firsthand accident stories. My time in shared labs and workspaces reminded me that one complacent team member puts us all at risk. Regular safety reviews, accessible safety data sheets, and simple signage help build a culture where everyone checks caps, spots leaks, and never shrugs off “just a tiny whiff.”
Dealing with chemicals in any lab or plant is about more than just trusting what’s printed on a safety data sheet. I’ve worked with silanes, and every time someone reaches for a bottle labeled 3-chloropropyldichloromethylsilane, there’s good reason to stop and check more than just the product code. This is not just any organosilicon compound—its reactivity offers advantages for synthesis, but also raises serious questions around compatibility and safety. It’s this experience, combined with evidence, that should shape how we use it.
I remember a colleague once underestimated the effect of moisture on this compound. Direct contact with water or even humid air causes a violent reaction, giving off hydrogen chloride gas. That’s not just an inconvenience; it’s a risk to everyone in the area. If you’re storing this chemical or using glassware that held water, count on corrosion, unexpected pressure build-up, and acid vapors. That’s not something you handle with a paper towel and move on from—it’s a problem that can bring the operation to a grinding halt and send people to the emergency room. Thorough drying of reaction vessels, tight seals, desiccators, or gloveboxes aren’t just fancy words—they’re lifelines.
Some labs get bold with their solvents. In my experience, inert solvents like dry toluene or hexane are worth every cent here, because unwanted reactions set in fast. Acetone, alcohols, and ethers go off with the compound just as eagerly as water, so it pays to skip cornershop improvisation. Add a strong acid or base to the mix, and the story ends with breakdown products you might not have planned for, including more corrosive gases and sometimes polymerized gunk that ruins equipment. Mixing this chemical, even accidentally, with sodium hydroxide or hydrochloric acid, for instance, sets off reactivity you don’t want in your facility—or your lungs.
Labs with large-scale apparatuses using metal lines, especially those not fully passivated, run into another problem. 3-chloropropyldichloromethylsilane tends to corrode certain metals, especially if any moisture is lurking inside the apparatus. That means even stainless steel can pit over time, compromising your instruments and quality control. In some cases, residues and byproducts accelerate these effects, which sneak up with costs nobody wants to pay.
Documentation helps, but real safety shows in habits. I’ve seen smart teams keep dedicated glassware just for chlorosilanes. They label and triple-check transfer vessels, and work only in ventilated fume hoods with spill kits and eye wash on hand. They never store the compound near incompatible chemicals or somewhere heat could encourage decomposition. Emergency planning goes beyond ticking off boxes—people drill responses, and personal protective equipment isn't negotiable.
Companies and researchers need steady education about the unique risks of mixing this silane. Engineering solutions include materials selection, better ventilation and enclosure systems, and online sensors for HCl and flammable vapor detection. When labs push chemistry forward with a respect for incompatibilities and a willingness to invest in equipment and training, everyone on the team goes home safe, and the science moves forward without preventable mistakes. Sometimes, progress means slowing down and giving incompatibility the respect it demands.
Anyone who’s spent time working in a chemistry lab or around industrial chemicals knows the stakes go way up with compounds like 3-chloropropyldichloromethylsilane. This stuff doesn’t just pose an irritation risk; it reacts violently with water, releases corrosive hydrochloric acid, and produces fumes nobody wants to breathe. Spilling it means potential burns and respiratory hazards, even for folks wearing gloves and goggles.
In my own experience, watching some people try to “dilute and drain” risky chemicals is like watching a crisis unfold in slow motion. 3-chloropropyldichloromethylsilane should never end up in a regular sink or trash bin. EPA guidelines make that clear, but more importantly, firsthand experience shows shortcuts in disposal often lead to emergencies that cost people their health and employers big fines.
Best practice for disposal starts with putting any unused chemical and contaminated items—gloves, paper towels, empty containers—in clearly labeled, sealed chemical waste containers. Storage before final disposal makes a big difference. Waste containers should use materials that can handle both the chemical and the fumes it releases. Metal pails corrode, regular plastic buckets crack. High-density polyethylene or lined metal cans do better. My old workplace used double containment, and we never had leaks or corrosion scares.
Most labs or factories don’t process this kind of waste on-site. Trained firms collect, transport, and destroy these hazardous chemicals under state and federal guidelines. I’ve seen environmental personnel come through like clockwork, donning respirators, double-checking manifests, and treating every transfer as a potential hazmat event—because history backed up their caution more than once.
Large-scale incinerators, run by waste contractors approved by the EPA or local authorities, neutralize dangerous compounds through high-heat combustion, reducing them to non-toxic ash and manageable off-gases. Scrubbing and filtration gear traps anything corrosive or toxic, keeping the pollution out of the air we all breathe. Without this technology, accidental poison gas incidents would be a lot more common. Incineration’s not a glamorous solution, but it works and keeps accidents off the news.
People working with chemicals like this deserve strong training. Every job I had with dangerous stuff began with hours of walkthroughs and simulation drills. Waste tracking logs, clear signage, and written handoff procedures didn’t just meet safety rules—they let everybody focus on the task, knowing backup plans covered unexpected spills or exposures.
Not every situation needs a dump truck and a caravan. For tiny spills and sample-sized leftovers, keeping a spill kit with absorbent pads and neutralizer makes things less scary. Some labs use activated charcoal or specific neutralizing agents under a fume hood, immediately containing vapors. After cleanup, every scrap and wipe still goes into the hazardous waste stream, not the regular trash.
Disposal routines don’t just protect the environment—they save time, cut medical bills, and keep production on schedule. Skipping steps with 3-chloropropyldichloromethylsilane risks more than regulatory trouble; it risks lives. Running a tight ship, keeping honest logs, and trusting expert waste contractors makes sure the only headlines come from scientific breakthroughs, not disasters.
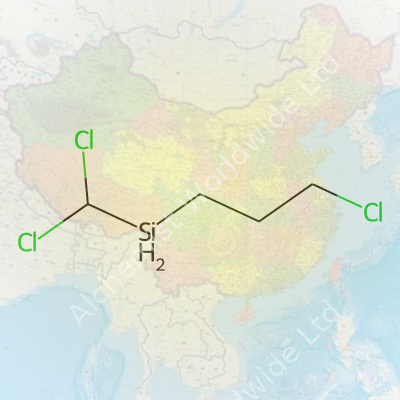
| Names | |
| Preferred IUPAC name | (3-chloropropyl)dichloro(methyl)silane |
| Other names |
3-chloropropyl(methyl)dichlorosilane 3-Chloropropylmethyldichlorosilane Silane, dichloro(3-chloropropyl)methyl- |
| Pronunciation | /ˌθriː-klɔːr.oʊ-ˈprɒp-il-daɪˌklɔːr.əʊ-ˈmɛθ.ɪl-ˈsɪl.eɪn/ |
| Identifiers | |
| CAS Number | 6297-41-6 |
| 3D model (JSmol) | `ClC(CCl)(Cl)CCCl` |
| Beilstein Reference | 1721441 |
| ChEBI | CHEBI:134737 |
| ChEMBL | CHEMBL3721966 |
| ChemSpider | 21631618 |
| DrugBank | DB14638 |
| ECHA InfoCard | 30fa11f6-5443-41e5-810b-1c07b8a2b01b |
| EC Number | 401-200-5 |
| Gmelin Reference | 108979 |
| KEGG | C18983 |
| MeSH | D016675 |
| PubChem CID | 71345372 |
| RTECS number | GV2450000 |
| UNII | 3AI8396ZT2 |
| UN number | UN3265 |
| Properties | |
| Chemical formula | C4H9Cl3Si |
| Molar mass | 218.48 g/mol |
| Appearance | Colorless to yellowish liquid |
| Odor | Pungent |
| Density | 1.307 g/mL at 25 °C (lit.) |
| Solubility in water | Decomposes in water |
| log P | 1.3 |
| Vapor pressure | 2.8 hPa (20 °C) |
| Acidity (pKa) | 14.5 |
| Refractive index (nD) | 1.482 |
| Viscosity | 1.809 mPa·s (25 °C) |
| Dipole moment | 1.79 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -427.6 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS06 |
| Pictograms | GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H314, H317, H318, H335, H411 |
| Precautionary statements | P210, P260, P280, P301+P330+P331, P303+P361+P353, P305+P351+P338, P309+P311 |
| NFPA 704 (fire diamond) | 3-2-2-W |
| Flash point | '64 °F' |
| Lethal dose or concentration | Lethal dose or concentration: LD50 (oral, rat): 1200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 2,620 mg/kg |
| NIOSH | L409 |
| IDLH (Immediate danger) | IDLH: 5 ppm |
| Related compounds | |
| Related compounds |
Trimethylsilyl chloride Triethoxysilane Dimethyldichlorosilane Methyltrichlorosilane Vinyltrichlorosilane Chloromethyltrimethoxysilane |