
Synthetic organosilicon chemistry opened up pathways for new surface modifiers, and 3-chloropropylmethyldiethoxysilane (CPMDES) made its debut during the second half of the twentieth century. Companies raced to develop cost-effective silanes for the emerging polymer and composite industries. The unique combination of the chloroalkyl group and silane functionality unlocked new adhesion methods, with German and US research labs reporting breakthrough trials by the 1970s. Years of iterative development refined purification and reaction control, after manufacturers realized trace impurities could wreck product performance. As industrial chemists grew more ambitious, CPMDES found use in new materials, hand-in-hand with the global demand for robust coupling agents.
3-Chloropropylmethyldiethoxysilane steps up as a clear or slightly yellow liquid designed primarily for use as a coupling agent, surface modifier, and intermediate. The compound stands out in the silane world for its dual reactivity: an organic chlorine that reacts with nucleophiles, and a pair of hydrolyzable ethoxy groups bound to silicon. Every bottle usually comes tightly sealed to block out atmospheric moisture, which can trigger unwanted hydrolysis and sluggish gel formation. Global distribution networks, especially in the United States, China, and Western Europe, guarantee widespread availability for research and manufacturing needs.
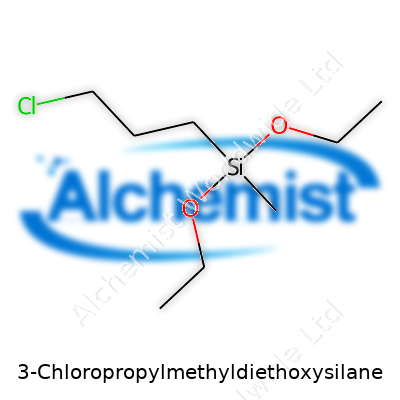
At room temperature, CPMDES holds as a colorless or light-yellow, volatile, and flammable liquid, with a density hovering near 0.97 g/cm³. Its chemical formula, C8H19ClO2Si, describes a three-carbon chain attached to a silicon atom, bearing methyldiethoxysilane functionality. With a molecular weight of around 226.77, CPMDES boasts a boiling point in the range of 224–226°C under atmospheric pressure, although it may decompose or hydrolyze with trace water vapor. Low water solubility contrasts with high miscibility in organic solvents such as toluene, dichloromethane, and ethers. Its vapor irritates mucous membranes, so proper ventilation and safe handling matter in every lab or factory.
Quality control relies on parameters like purity (often above 97%), refractive index (nD25: 1.410–1.420), and maximum allowed levels of acid and water content (generally below 0.1%). Bottles arrive labeled with manufacturer name, batch number, shelf life, and safety hazard symbols. UN shipping classifications and local regulatory codes appear on every drum, combined with hazard and precautionary statements as dictated by GHS. Chemical suppliers ship with data sheets that map out basic hazards, recommended storage temperatures (preferably below 30°C), and instruction to keep containers tightly sealed against air and humidity. Storage away from strong oxidants and bases minimizes risk of runaway exotherms or degradation.
Production of 3-chloropropylmethyldiethoxysilane starts from methyltrichlorosilane, which reacts with ethanol under controlled conditions to yield methyldiethoxysilane. Next comes hydrosilylation: the insertion of 3-chloropropene across the Si-H bond, catalyzed by a platinum complex or other transition metals. Careful distillation and vacuum stripping remove excess reactants and allow isolation of the target silane. Manufacturers monitor and adjust steps since hydrolysis or side reactions (especially unwanted ether cleavage by strong acids or base) can dent product yield and trigger wasteful byproducts. Skilled operators interpret temperature and pressure data on the fly to maximize output.
The compound shines as a customizable platform for further synthesis. The terminal chloro group provides a gateway to nucleophilic substitution with amines, thiols, or alkoxides, allowing new functionalities to be tethered to the silicon backbone. Hydrolysis of the ethoxy groups, when catalyzed by acid or base, generates reactive silanols, which then condense with hydroxylated surfaces or themselves to create robust Si–O–Si covalent linkages. This opens doors for glass, metal, or ceramic surface anchoring, with the organic moiety offering unique attachment points for further molecular engineering. Applications in the manufacture of functional polymers depend on these modifications, driving product diversity.
Chemists often encounter CPMDES under these alternative labels: 3-chloropropyl(methyl)diethoxysilane, 3-chloropropyldiethoxymethylsilane, and diethoxy(methyl)(3-chloropropyl)silane. International trade employs CAS Registry Number 13501-76-3. Major suppliers provide their own SKUs and trade names but stick to clear chemical nomenclature for regulatory compliance and supply chain traceability. Accurate labeling simplifies logistics, procurement, and communication across laboratories and industrial sites.
Long experience in industrial labs underlines the importance of rigorous handling protocols. Inhalation or skin contact can cause irritation, especially if the compound hydrolyzes right on exposed tissues. Appropriate personal protective equipment, including goggles, nitrile gloves, and chemical-resistant overcoats, mark the starting point for safe practice. Good engineering controls, such as fume hoods or local extraction, handle typical vapor levels. Spill management counts on absorbent materials and immediate cleanup, never neglecting the need for proper waste containers for both solid and liquid residue. Regular safety audits focus on chemical inventory accuracy, careful recordkeeping, and clear lab signage.
Real-world use cases span several sectors. The compound acts as a go-to coupling agent to promote adhesion between organic polymers and inorganic fillers, particularly in the composites and plastics industries. Laminates, wire and cable coatings, adhesives, and sealants all take advantage of its ability to establish a solid bridge at the molecular level between otherwise incompatible phases. High-performance paints, resins, and coatings depend on silane treated surfaces for extended service life, reduced cracking, and fatigue resistance. Companies looking to tweak surface tension or create discrete functional layers deploy CPMDES in surface modification workflows. Some research groups also employ the substance to create precursors for ionic liquids, silsesquioxanes, or organofunctional siloxanes, each with niche commercial or scientific utility.
Active R&D programs push the boundaries of silane coupling chemistry, with CPMDES frequently featured in academic publications that explore new adhesive technologies, nanocomposite production, and biocompatible surface engineering. Researchers test how the compound performs under thermal cycling, solvent immersion, or sustained mechanical load. Analytical techniques such as FTIR, NMR, and XPS clarify reaction mechanisms and product profiles. The drive to lower VOC emissions from processing steps has prompted new methods for deploying silanes with minimal residual solvent. Start-ups and established players alike collaborate with academic labs to pilot advanced surface treatments for electronics, automotive parts, and construction materials. Standard test methods assess shear strength, water uptake, and resistance to environmental stress cracking in end products.
Toxicologists have mapped out the hazards connected to CPMDES, drawing on data from animal studies and accidental exposures. Acute toxicity appears moderate, with discomfort or damage mostly linked to eye, skin, or lung irritation. Repeated high-dose contact can influence the liver or kidney in rodent models. Hydrolyzed silanes may generate ethanol and hydrochloric acid as byproducts, introducing secondary risk in poorly ventilated environments. Sensible practitioners refer to material safety data sheets and stay alert to emerging research on chronic effects or environmental persistence. Ongoing surveillance seeks to clarify breakdown pathways and identify any hazardous long-lived intermediates, critical for lifecycle assessment and green chemistry compliance.
Demand for advanced silane coupling agents continues to climb as global industries push for lighter, more durable, and better-integrated composite materials. Researchers are scrutinizing the scalability and environmental footprint of CPMDES synthesis, targeting lower energy consumption and minimal waste. Next-generation polymers, smarter sensors, and improved biomedical devices all require surface modifications that traditional means cannot match. Regulatory pressures focus attention on the safe use, lifecycle tracking, and eventual disposal or recycling of organosilanes. Companies investing in renewable raw materials and solvent-free processes stand out as leaders in meeting these sustainability and performance challenges.
Most people have never heard of 3-Chloropropylmethyldiethoxysilane, but you’d be surprised how much it shapes a lot of the things we use and depend on. The real interest in this chemical comes from the way it bridges organic and inorganic materials. I’ve spent years working around coatings, construction, and adhesives, and I keep seeing this silane pop up at the center of improvements in bonding and durability.
Try gluing glass and plastic or metal and rubber without the right chemistry in place. Nothing really seems to hold. Here’s where 3-Chloropropylmethyldiethoxysilane steps in. This silane functions as a coupling agent. It stands between two very different worlds—something organic like a plastic polymer and something inorganic, often a mineral surface. Once applied, it improves how well adhesives, paints, or sealants grip onto things like glass fibers, ceramics, or metals.
Silicone sealants last longer and hold tighter because of this molecule. Automobile parts, electrical insulation, and construction materials owe their integrity to its use. I’ve watched glass manufacturers dip fiber bundles in silane solutions before weaving them into fiberglass insulation. The result? Stronger, weather-resistant products that don’t break down in damp or cold conditions.
Modern industry relies on composite materials to keep things both lightweight and tough. 3-Chloropropylmethyldiethoxysilane plays a major part here. Chemically, it reacts at both ends: one end latches onto the inorganic stuff (like glass, stone, or sand), while another end grabs hold of certain resins. This lets manufacturers create smoother, stronger, and more reliable composites for wind turbine blades, boats, aerospace parts, and sports gear.
Silanes like this one help cut down on cracking, delamination, and moisture leaks. Each of these problems can cost millions if they show up in large-scale building or transportation projects. In my years consulting for manufacturers in the energy sector, these silanes have saved headaches by stretching the life of expensive components.
Bringing out the best in any industrial additive raises questions. 3-Chloropropylmethyldiethoxysilane works wonders but isn’t something you want splashed on bare skin or down the drain. Mishandling silanes poses risks to both worker safety and the environment. Companies keep safety data sheets at the ready and require workers to wear gloves, goggles, and respirators for good reason. Health agencies like OSHA and the EU’s REACH program call for strict tracking and reporting on substances like this to ensure exposure stays low.
Some makers now look for safer alternatives or greener production methods to further shrink risks. Swapping in bio-based materials or tweaking the process to trap more fumes and waste before they hit the outside world are both on the table. Over time, tighter safety rules and smarter chemistry promise to keep benefits high and hazards low.
Without 3-Chloropropylmethyldiethoxysilane, the bond between so many everyday materials would weaken. The bridges, cars, phones, and countless other products in our lives show the results. Engineers and chemists keep finding new, smarter ways to use this coupling agent. The real trick is staying persistent about safety and efficiency, always watching for better and cleaner ways forward.
Working around chemicals like 3-Chloropropylmethyldiethoxysilane means folks need to take precautions seriously. This clear liquid may seem harmless in a tightly capped drum, but its vapors and the possibility of reactions turn it into a different story. Exposure—mainly through skin or inhalation—hits hard. Even experienced handlers can slip up without the right habits in place.
I once watched a colleague rush through their storage checks. One missed whiff, and they landed in the first-aid station with eye irritation and a bad cough. That quick shortcut set back the work for days. The lesson sticks: consistent and careful handling protects everyone.
If you keep 3-Chloropropylmethyldiethoxysilane around, put it in a well-ventilated storage area. Forget windowless closets or places that get warm—they let fumes build up, raising the risk for anyone nearby. Drums or containers should resist corrosion and offer tight lids, keeping the air clean and the product stable.
Heat and open flames set up a ticking time bomb. This chemical catches fire and breaks down in the wrong conditions. Folks who store it near heaters or forklifts carrying anything sparking are asking for trouble. Room temperature works best, far from direct sunlight or any spot that swings much warmer or cooler than the rest of the facility.
Chemical splash goggles and gloves make a difference every single time. I’ve heard too many old-timers say they’re careful, only to see them wiping sanitizer in tears after a splash. Nitrile gloves hold up well, and face shields stop the stuff from getting in your eyes or on your skin, even during a spill or quick transfer.
If you need to pour or mix, use a fume hood or directed exhaust. Someone opens a drum without venting, and even a short cloud of vapors gets into the lungs fast. Make sure there’s easy access to eyewash stations and showers, not just signs taped on the walls nobody reads.
Labels matter. I’ve walked through labs where someone relabeled a reused bottle with a black marker, and no one caught it until it ate through the plastic. Clear, chemical-resistant labels stand up to splashes and time, making sure no one guesses about what’s inside. Spill kits and containment materials should stay close by—absorbent pads, neutralizers, and sealed waste bins let any worker handle small emergencies before they get out of control.
Managers boost safety by backing up regular training, not just at hiring but throughout the year. Share real stories, keep safety data sheets in easy reach, and run drills. OSHA tracks incidents where missing training led to injuries, and insurance claims soar when a team gets complacent.
People think regulations just slow things down, but those rules came from real mishaps. Responsibly storing and handling 3-Chloropropylmethyldiethoxysilane isn’t about ticking boxes; it’s about making sure folks walk out healthy at the end of the day. Taking pride in safe processes, wearing the right gear, and paying attention keeps families from getting bad news.
Plenty of chemicals flow through factories and labs that most people can’t pronounce. 3-Chloropropylmethyldiethoxysilane falls squarely in this category. I’ve spent years working with people in coatings and plastics, where reagents like this one help bind things together and improve adhesion. Even for those who use it directly, safety data sheets wind up feeling like a blur of warnings, numbers, and symbols.
People wonder if contact with this silane can do real harm. Strong evidence says yes. Direct contact with the liquid may burn skin or eyes, and inhalation causes throat and lung irritation. Reports from chemical plant workers highlight headaches, nausea, and breathing difficulties after accidental exposure. If splashed into the eyes, studies have shown rapid inflammation, sometimes leading to long-term sensitivity. The chlorine atom in its structure increases its potential for causing harm compared with silanes without reactive halogens.
Animal studies show even small doses over time can harm lungs and liver, though long-term data in humans remains limited. The ethoxy groups react with moisture, including that in lungs—a fact I learned during a plant shutdown accident, where improper cleanup put colleagues at risk. Quick response and the right protective gear saved the day, but the incident drove home the chemical’s danger outside of controlled settings.
3-Chloropropylmethyldiethoxysilane doesn’t just vanish after use. Spills in factories can seep into soil, where the compound slowly breaks down, but not before impacting water quality. I once watched a team frantically build containment berms around a leak, working to keep the run-off out of a drainage ditch that fed local wetlands. Chlorine-containing compounds, in particular, create a risk for aquatic life—studies show damage to the gills and organs of fish at low concentrations.
The material reacts quickly with water, generating ethanol and hydrochloric acid. That reaction lowers pH and stresses anything living downstream. Wastewater treatment facilities see these spikes in acidity and have to neutralize them. Some municipalities report periodic spikes in chlorinated organics that can trace back to specialty chemical use by industry.
Safety training and the right equipment form a solid first line of defense. Nitrile gloves and face shields make a big difference. I encourage every workplace storing this compound to enforce clear labeling and spill kits at arm’s reach, since confusion and delay fuel most accidents I’ve seen. Fume hoods capture escaping vapors, and good ventilation means fewer workers will ever get exposed.
Disposal brings another challenge. Leaks at waste handling sites magnify environmental risks, so pretreatment and strict adherence to disposal rules matter. Some companies reclaim unused silane to cut waste and costs—a move I’ve seen lower both spills and expenses. On a bigger scale, pressure from communities and regulators for greener alternatives spurs research into silanes that do the same job without chlorine groups.
Earning trust around chemicals like 3-Chloropropylmethyldiethoxysilane takes transparency, respect for proven dangers, and a push for better safety measures at every step. You can’t see or smell this stuff at dangerous concentrations, so relying on monitoring and early warnings isn’t just smart—it’s essential. Staying informed and fixing gaps early keeps both people and the environment safer, even as chemical processes grow more complex.
Talking about chemistry often brings back those high school lab memories, where safety goggles fogged up as soon as you pulled them on. A compound like 3-Chloropropylmethyldiethoxysilane carries enough syllables that it might make your eyes glaze over, but breaking down the formula—C9H21ClO2Si—shows there’s something pretty practical going on here.
This molecule looks about like you’d expect for a silane: you get a silicon center, two ethoxy groups, a methyl, and a three-carbon chain capped off with a chlorine atom. On the benchtop, as with plenty of similar silanes, it turns up as a colorless to light yellowish transparent liquid. It does not smell pleasant. No surprise there. Chemical factories don’t win perfume awards, and this compound serves as a reminder.
The value in seeing the real structure isn’t just for folks in lab coats. The ethoxy groups attach to silicon, and in the right conditions, these break apart and bond with lots of other surfaces—glass, metals, even plastic. That’s not a textbook definition; it’s practical magic for chemists and manufacturers. Because of this, industries use it to “couple” materials together. You can glue glass fibers inside plastic car parts so they don’t come apart in a crash, or improve electronics so they survive harsh environments. It’s straightforward: the right functional group on a silane like this means surfaces that don’t stick together suddenly get along just fine.
Looking past the textbook, there’s a risk here. The chlorine atom on the propyl chain brings reactivity. Anyone who’s handled alkyl chlorides knows skin contact, inhalation, or even the wrong kind of slight spill causes more trouble than it’s worth. It’s not just about safety sheets nobody reads; experience in the lab means knowing what to avoid. Proper storage matters. Seal it away from moisture and store it under nitrogen or dry air—humidity turns these silanes into sticky messes, releasing corrosive vapors nobody wants in their lungs.
With all the talk around chemical safety in the news, handling chemicals like this responsibly is about E-E-A-T for real. Good experience and expertise demand double-checking the facts—does the supplier provide recent Certificates of Analysis? Can you independently confirm composition with NMR, IR, or gas chromatography? Trust doesn’t come from a nice label.
From an environmental standpoint, regulations on organosilanes keep tightening as their toxicity in aquatic systems becomes better understood. Not disposing of waste properly can lead to fines or, worse, lasting damage to water sources. I’ve seen engineers shrug off a ‘little rinse’ down the sink, but the downstream effects are real. Each lab spill and improper dump adds up.
Beyond training and proper PPE, introducing closed-system dispensers or automated pumps cuts exposure. Labs and factories that invest in these safety features see fewer incidents. Companies also increasingly build oversight into standard processes, documenting not just storage and disposal, but staff training too. For smaller organizations, working with trusted suppliers and external consultants plugs experience gaps.
In the end, asking the right questions about the material—what exactly is it, what could go wrong, how do I keep people and the planet safe—keeps us on the right track. Keeping it real and respecting both the science and the risks brings chemicals like 3-Chloropropylmethyldiethoxysilane out of the shadows and into safe, practical use.
Anyone who’s spent time with specialty chemicals like 3-Chloropropylmethyldiethoxysilane knows just how tricky the logistics can get. This isn’t a harmless product. Even routine handling without proper management turns into a potential health and environmental hazard in a flash. When a transport container leaks—maybe during a bumpy highway run—exposure risks shoot up, so good practice kicks in well before anything leaves a loading dock.
Stories from the supply chain show what can go wrong. Chemical burns, lung issues, and fire hazards have actually reshaped company protocol over the years. Direct skin or eye contact with this silane irritates or damages tissue. Inhalation risks grow in enclosed spaces, especially if vapors escape.
Safety Data Sheets (SDS) published by trusted chemical companies lay it out: this silane reacts with moisture, giving off corrosive byproducts like hydrochloric acid. Mixed with water or steam—whether from a broken valve or a rainy day—danger escalates. Trucks and containers exposed to weather without proper sealing leave workers scrambled and local communities on edge. So, solid packaging and weatherproofing are just as vital as the product inside.
Laws shape much of the journey. The U.S. Department of Transportation (DOT) and similar groups in other countries assign this chemical into hazard classes, usually requiring labels and sealed drums. Rules push companies to mark tanks with proper placards, fill out detailed shipping papers, and give drivers clear instructions. These aren’t empty demands; they’ve come from past emergencies—like chemical fires or unreported leaks—where missing paperwork delayed emergency response. Written procedures and visible labeling matter in a real crisis.
No safety protocol succeeds without skilled workers. Routine employee training familiarizes the team with personal protective equipment, emergency procedures, and the right steps to take if a spill happens. Shortcuts—switching out goggles for sunglasses, skimping on gloves—lead to real injuries. A seasoned colleague of mine used to say the best insurance for chemical transport isn’t paperwork or insurance—it's well-trained people keeping their eyes open.
Transport teams lean toward steel drums with inner linings or high-grade plastic containers to fend off corrosion and accidental leaks. Reliable gaskets, sealed closures, and secure strapping keep impacts from cracking a container in transit. Some fleets install constant in-cabin monitoring and vapor detectors. This isn’t just about ticking boxes—it’s about turning vehicles into safe bubbles on the road.
Practical improvements start with communication. Shippers and carriers must review current regulations and company policy together. Before the wheels turn, a checklist covering containers, labeling, and emergency supplies keeps preventable errors out of the picture. Regular audits and surprise checks spot weak links early.
Another smart step: invest in digital tracking. RFID tags, GPS updates, and electronic shipping documentation support team coordination and make sure emergency responders get vital information on the spot. These tools came out of real mishaps—fire departments stuck guessing at spill content lose critical minutes.
I’ve watched companies add “safety days,” where teams share field stories and test real-life scenarios. The most memorable sessions didn’t use slides—they let workers face simulated spills in controlled conditions.
Safe transportation isn’t built on hope or checklists alone. It grows from an honest look at risk, respect for every hand involved, and a willingness to invest in sturdy gear and continuous training. Every avoided accident adds one more reason to keep raising the bar in chemical logistics.

| Names | |
| Preferred IUPAC name | 3-chloropropyl(methyldiethoxy)silane |
| Other names |
3-Chloropropyl(methyl)diethoxysilane 3-Chloropropylmethyldiethoxysilane 3-Chloropropyl(methyl)diethoxysilane Diethoxy(methyl)(3-chloropropyl)silane Silane, (3-chloropropyl)diethoxy(methyl)- |
| Pronunciation | /ˈθriː-klɔːr.əˌproʊ.pɪlˌmɛθ.iˌdaɪˌɛθ.ɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 3049-70-7 |
| Beilstein Reference | 2368736 |
| ChEBI | CHEBI:152606 |
| ChEMBL | CHEMBL1778821 |
| ChemSpider | 21160023 |
| DrugBank | DB14441 |
| ECHA InfoCard | 03e3ac51-c63c-4d70-bcf6-218b944e4c5c |
| EC Number | 203-934-4 |
| Gmelin Reference | 87734 |
| KEGG | C19229 |
| MeSH | 3-Chloropropylmethyldiethoxysilane |
| PubChem CID | 87155 |
| RTECS number | GV8975000 |
| UNII | JC3R9GKE9M |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C9H21ClO2Si |
| Molar mass | 210.77 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Pungent |
| Density | 0.956 g/mL at 25 °C |
| Solubility in water | insoluble |
| log P | 1.9 |
| Vapor pressure | 1 mmHg (25°C) |
| Acidity (pKa) | 12.7 |
| Refractive index (nD) | 1.4140 |
| Viscosity | 3.5 mPa.s |
| Dipole moment | 2.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 390.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H302, H314 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-1-W |
| Flash point | Flash point: 72 °C |
| Autoignition temperature | 245 °C (473 °F; 518 K) |
| Lethal dose or concentration | LD50 Oral Rat 2246 mg/kg |
| LD50 (median dose) | LD50 Oral Rat 2410 mg/kg |
| NIOSH | No data. |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Chloropropylmethyldiethoxysilane: Not Established |
| REL (Recommended) | 10 ppm (60 mg/m3) |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Chloropropyltriethoxysilane 3-Chloropropyltrimethoxysilane 3-Chloropropylmethyldimethoxysilane Methyltriethoxysilane Propylmethyldiethoxysilane 3-Chloropropyltriethoxysilane |