
Watching the evolution of organosilicon chemistry feels like seeing a fundamental shift in the way industries treat their surface science. Scientists searching for durability in coatings and adhesion performance have leaned on silane coupling agents for decades. The commercial introduction of derivatives like 3-Chloropropylmethyldipropoxysilane carried out a gradual march out of dusty academic laboratories and into full-scale industrial production. Chemical companies responded to demands from plastics, rubber, and advanced materials fields during the mid-to-late 20th century by tweaking alkoxy silanes, adding functional groups, and expanding applications. Before the digital era made technical information widely available, specialists had to rely on laborious trial and error in both synthesis and quality control, laying the foundation for the reliable, high-purity compounds traded today.
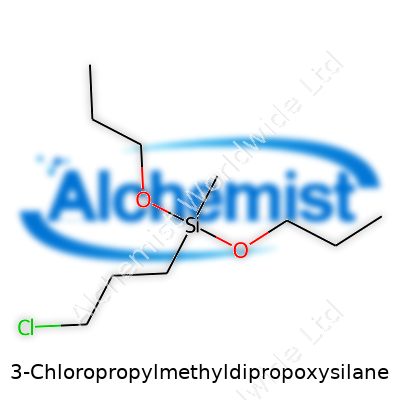
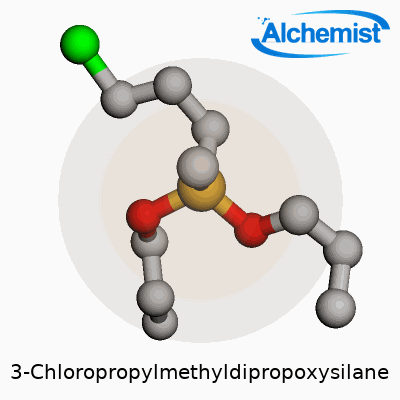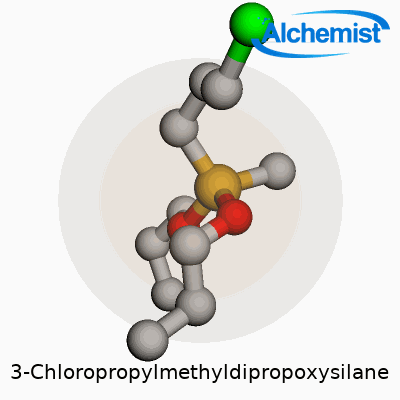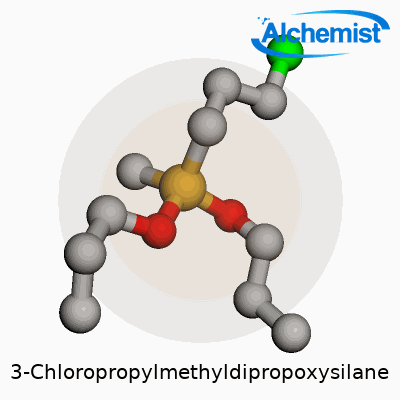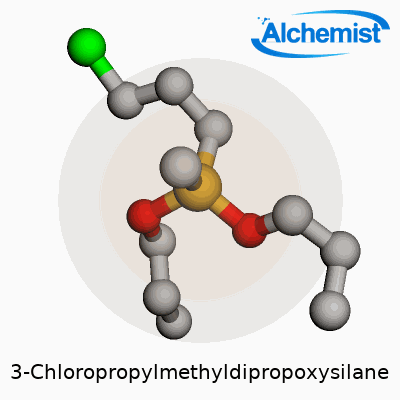
3-Chloropropylmethyldipropoxysilane stands out among organosilicon compounds due to its ability to offer reactive handles at both silicon and carbon ends. The molecule brings together a methyldipropoxysilane backbone with a 3-chloropropyl group, resulting in a clear or faintly yellow liquid that blends flexibility with performance. This dual nature enables it to serve as a powerful cross-linker, adhesion promoter, and chemical intermediate in a host of production environments from adhesives to specialty coatings.
A bottle of 3-Chloropropylmethyldipropoxysilane typically holds a liquid with low viscosity and a boiling point above 200°C. You’ll notice a sharp odor characteristic of many chlorinated organics. The density comes in around 0.98 g/cm³, making it easy to handle through standard dosing pumps or dispersion systems. Chemists appreciate its propoxy groups, which hydrolyze in the presence of water, forming silanols that can bond strongly to glass, metal oxides, and minerals. The chloropropyl group attracts attention for its tendency to undergo substitution and elimination reactions, one reason why this silane frequently appears in research focused on surface modification and polymer integration.
High-value manufacturing relies on clear, reliable technical data to ensure that each batch delivers on its promise. Typical labeling lists molecular formula as C13H29ClO2Si, with CAS number 94442-22-5. Purity standards often exceed 96%, controlled moisture below 0.5%, and GC area normalization for trace impurities. Shipping standards line up with UN transport codes for flammable liquids, emphasizing careful handling and traceability of lot numbers. Companies stake their reputation on reproducibility, so stringent tracking from synthesis to storage plays a crucial role. SDS documentation highlights the need for PPE, ventilation, and disposal protocols to keep operators and end-users out of harm's way.
Lab synthesis of 3-Chloropropylmethyldipropoxysilane usually begins with alkoxylation of methylchlorosilane using n-propanol under controlled reflux, followed by nuanced chloropropyl group introduction, either via substitution or direct Si–C functionalization. Industrial reactors scale this up through continuous feeding, tight temperature controls, and fractionation columns that separate side-products. This pathway calls for trained technicians who understand both the hazards of chlorinated silicones and the importance of eliminating trace acids that could degrade product quality or catalyst life in downstream reactions.
One advantage in the toolbox is the reactivity of the chloropropyl arm, which undergoes nucleophilic substitution to yield amines, thiols, and other functional groups. Researchers and process engineers often graft 3-chloropropyl derivatives onto silica, alumina, or other filler surfaces, customizing compatibility with organic and inorganic phases. The silane's propoxy groups activate under acidic or basic hydrolysis to generate silanol intermediates, forming covalent Si–O–Si bridges at interface boundaries. These bonds don’t just improve mechanical strength; they often push thermal and chemical resistance to the next tier, especially under harsh environmental conditions.
Find 3-Chloropropylmethyldipropoxysilane under trade names in catalogs ranging from multinational suppliers down to boutique chemical shops. Synonyms span from 3-chloropropyl(methyl)dipropoxysilane to 3-chloropropylmethyldipropoxysilane, and sometimes technical short hands like CPMS-DPS. Manufacturers occasionally specify the propoxy variant for clarity, given the existence of similar methoxy- or ethoxy-functionalized silanes in parallel sectors. Clear nomenclature and batch traceability remain cornerstones for any R&D or production setting, avoiding confusion and error in specifications.
Synthetic chemists and plant personnel working with chlorinated silanes appreciate the balance between utility and risk. Fumes irritate the eyes, throat, and lungs, and liquids attack skin and mucous membranes. Facilities routinely design local exhaust ventilation, use chemical splash goggles and gloves, and monitor storage areas for leaks or exotherms. Industry follows REACH, GHS, and OSHA guidelines, requiring training, spill preparedness, and thorough documentation. Waste has to be destroyed in incinerators with flue gas scrubbing, given the organochlorine content’s potential for forming hazardous by-products. Companies failing to update safety protocols risk not only regulatory fines but also eroding the workforce’s trust—something no technical breakthrough can repair.
3-Chloropropylmethyldipropoxysilane has become a mainstay in sectors looking for durable surface bonds. Rubber manufacturers adopt it to enhance filler dispersion and build stronger siloxane linkages inside tires and gaskets. Coating plants treat glass and metal surfaces with this silane to boost water repellency and paint adhesion, stretching the lifespan of everything from architectural panels to windshield laminates. Electrical insulation engineers turn to organosilanes for their hydrophobic boundary layers, reducing moisture-driven failure rates in cables or connectors. This molecule doesn’t operate as a magic bullet, but its role at the interface edge drives up product value in a fiercely competitive marketplace.
Ongoing research explores new ways to unlock the potential of silane compounds, including 3-Chloropropylmethyldipropoxysilane, in fields like nanotechnology, biomedical devices, and sustainable composites. Labs experiment with modified silanes for self-assembling monolayers, responsive materials, and high-performance elastomers. Researchers probe reaction kinetics, long-term stability, and compatibility with next-generation matrices, all of which help push past prior limits in mechanical and thermal properties. Industry and academia often collaborate here; open data sharing, standardization efforts, and pre-competitive consortia speed up the feedback loop between discovery and commercial impact.
Robust toxicity studies flag the principal risk in repeated lung, eye, or dermal exposure. Animal models show irritation at moderate doses, with chronic testing zeroing in on reproductive and organ toxicity endpoints to satisfy global chemical safety boards. Labs also track breakdown products; in water, hydrolyzed fragments can persist in the environment or react into secondary pollutants. These findings shape regulatory controls along the supply chain, reinforcing the duty of suppliers to disclose hazards and help downstream users manage risks. The shift toward green chemistry places extra pressure on both industry and regulators to keep tabs on emerging toxicity data, ensuring continued confidence in use.
New applications on the horizon expand beyond legacy coatings and elastomers, as silane chemistry enables breakthroughs in electronics, medical implants, and engineered surfaces designed to clean themselves or resist fouling. The push for green production methods—cutting volatile emissions, recycling solvents, and inventing more benign hydrolysis routes—will re-shape both plant floors and R&D targets. End users demand faster curing, lower temperature processes, and higher reliability, challenging chemists to squeeze every bit of performance from functionalized silanes. Transparent supply chains and digitalized documentation add trust and speed to market, letting silane innovations find their footing wherever adhesion, durability, or surface modification tip the balance.
3-Chloropropylmethyldipropoxysilane shows up in so many manufacturing settings and chemical processes. Most people never see the stuff up close, but if you’ve worked in adhesives, coatings, or specialty plastics, you probably heard about it. The chemical stands out for its ability to create a bridge—making one thing stick well to another. This isn’t some theoretical benefit. It matters when composite materials need to stay together, or paints need to hold tightly to tricky surfaces.
In factories making glass fiber products or high-performance plastics, 3-Chloropropylmethyldipropoxysilane brings value by helping these materials blend their unique strengths. As an organosilane, it holds special talents for linking organic polymers with inorganic fillers. This means fiberglass-reinforced plastics end up tougher and longer-lasting. It’s hard to ignore what that brings to wind turbine blades or car parts constantly exposed to weather and wear.
Anyone working with high-strength adhesives understands the challenges of making things stay put. Moisture, heat, and vibration all try to break bonds apart. 3-Chloropropylmethyldipropoxysilane tackles these problems by taking part in the chemical bonding process. It lays down a foundation so adhesives don’t just sit on surfaces; they lock in. In my experience around epoxy resin projects, a silane like this changes the story from “this might come loose someday” to “good luck prying this off.”
Paint and coating companies keep a close eye on silane coupling agents such as this one. Without them, coatings that look flawless in the factory might start flaking after a single wet season. 3-Chloropropylmethyldipropoxysilane serves as a primer at the molecular level. It bonds to both the substrate and the coating, stopping water, salt, and other troublemakers from sneaking underneath. I’ve seen anti-corrosion treatments that relied on this chemical stand up for years where cheaper options failed after only a few months.
Tire makers and other rubber product specialists draw from a familiar toolkit, and silanes show up regularly on the workbench. By linking rubber to mineral fillers, 3-Chloropropylmethyldipropoxysilane helps boost stretch and strength. A little goes a long way. It isn’t just about making rubber tough; it’s about making it last through thousands of cycles on the road or in machinery. Automotive engineers know skipping on this step just ends up costing more in the long run due to tire failure or poor performance.
There’s more conversation these days about responsible chemical use. 3-Chloropropylmethyldipropoxysilane, like many industrial chemicals, demands proper handling. Producers now provide better training and clear labeling to lower risk in the workplace, and plenty of innovation focuses on lowering emissions and waste. Regulatory checks in the US, Europe, and Asia keep the worst practices out, but it still takes a real commitment from companies to maintain high standards and keep workers safe. Moving towards greener substitutes will take time, but ongoing research encourages positive change without giving up performance.
No one wakes up thinking about silane chemistry, but its benefits ripple into everyday life. Cars last longer, buildings hold together, electronics work even in harsh conditions. As industries push towards sustainability and fewer repairs, 3-Chloropropylmethyldipropoxysilane hangs around for a reason—reliability built on real-world chemistry. Better transparency, education, and mindful use can push progress even further. In the end, what matters most is the safety, durability, and innovation these materials support.
3-Chloropropylmethyldipropoxysilane often shows up under the formula C10H23ClO2Si. Digging into its structure helps clarify this: there’s a silicon atom at the core, surrounded by two propoxy groups, one methyl group, and a three-carbon chain capped with a chlorine atom. Put the pieces together and it stacks up to 10 carbons, 23 hydrogens, one chlorine, two oxygen atoms, and a single silicon atom.
To calculate the molecular weight, it makes sense to use the standard atomic masses from the periodic table:
Add it up and you get about 238.83 g/mol. This number guides research chemists as well as industrial users—dosage precision and safety calculations depend on it.
Having worked with silanes in the lab, I can say that their structure steers everything from reactivity to handling needs. The chloropropyl arm gives it a reactive anchor, making this molecule stand out for surface treatments and as a coupling agent in materials science. Two propoxy groups help boost compatibility with organic compounds. The methyl group brings stability, helping prevent unpredictable side reactions.
In adhesives, coatings, and sealants, chemists look at molecules like this—one slip in the formula or calculation and the final product’s properties shift. If a batch goes wrong, costs spike and safety could get compromised. Laborious as it sometimes feels to double-check the math, those checks stop mistakes before they spread downstream.
Industries lean on these organosilanes to optimize performance or bridge gaps between incompatible materials like glass and polymers. Long-term success in those applications spins off the chemical backbone and total weight—the molecular weight affects how much you need for an exact dosage, and the formula influences reaction pathways. Larger scale facilities or smaller custom shops both face the same kind of problems, especially on scaling up or chasing down contaminants in a product batch.
Across research and regulatory work, E-E-A-T (Experience, Expertise, Authoritativeness, and Trustworthiness) drives decisions. In my own time developing composite coatings, using the published formula and molecular weight proved crucial for reproducibility and regulatory compliance. Chemical traceability, transparent lab records, and access to up-to-date data all flow from sound knowledge of a compound’s building blocks.
As new rules roll out around the world, traceability gets more crucial. Chemicals like 3-Chloropropylmethyldipropoxysilane need to be identified quickly and accurately, with the right weights at each step of the supply chain. Updated databases and ongoing training serve as a major solution: lab workers, procurement staff, and compliance officers all benefit from easy access to trusted references. Reducing data errors helps smooth out regulatory checks and keeps both users and the environment safer. Solid, precise chemical data pays off each time someone reaches for a bottle in the lab or scales up for production.
Chemicals like 3-Chloropropylmethyldipropoxysilane show up in plenty of industrial settings—from adhesives and plastics to coatings and sealants. I remember my early days in the lab, how easy it was to overlook basic handling steps in a rush or under pressure to cut corners. Over time, seeing coworkers fall ill or hearing stories of accidents convinced me every extra moment spent storing or handling substances like this one safely pays off. In places with ventilation issues or poor labeling, health risks can escalate quickly.
Good habits build safety culture. For liquids prone to hydrolysis, I kept them in cool, well-ventilated, dedicated chemical storage cabinets. These cabinets stayed far from heat. I double-checked caps—no loose tops, no crusts, and if I saw a damaged container, immediate replacement meant fewer headaches later on. Experience taught me storing this kind of silane away from water supplies, acids, and oxidizers is critical since vapors react with moisture and could form hydrogen chloride.
Labels must be clear. Nobody wants to reach for methyl isobutyl ketone and accidentally grab silane. Every new batch got a fresh label with arrival date. If outdoor storage cropped up, I used secondary containment—plastic trays to catch leaks, keeping site managers and environmental folks happy.
Most incidents come from routine tasks. The guy who skips gloves or the coworker who forgets their goggles during a transfer can wind up in the infirmary. Splashing silane on skin might not seem serious at first, but irritation, burning, and longer-term issues often show up hours later. In my own hands-on work, I chose nitrile gloves—latex just didn’t stand up. Faceshields beat safety glasses if you’re pouring or sampling.
Respirators become as important as gloves in some labs, especially without engineering controls like fume hoods. I saw a technician cough and choke at a bench with a barely controlled bottle inches away; investing in portable fume extractors eliminated that risk. Work areas need quick access to eye wash stations and emergency showers—old ones often get cluttered, and in a real spill, seconds count.
Mistakes teach tough lessons. A coworker once stored a drum near a steam pipe. Foggy room, warm conditions, and suddenly, white fumes pooled across the floor. The cleanup involved hazmat suits, phone calls to city authorities, and a $1,000 dollar fine. That memory stuck.
People who handle 3-Chloropropylmethyldipropoxysilane every day get comfortable until a missed safety step proves costly. Weekly site safety walks uncovered spilled beads, cracked vials, and missing spill kits. It took management support to get everyone retrained and paperwork updated. After that, incidents dropped fast.
Success comes from repeated reminders, clear rules, and making life easy for workers to do the right thing. Good training works better than another sign on the wall. Tighter checklists, never cutting back on PPE, and simple rules about who can access hazardous materials go a long way. Electronic records keep track of inventory and expiration, which prevents forgotten chemicals from sitting on shelves for years.
People new to the field benefit from mentorship. Letting green staff shadow experienced handlers builds confidence quicker than slideshows. Frequent drills, from spills to burns, turn safety into instinct. Involving everyone in weekly reviews, even the ones who roll their eyes, changes habits over time.
3-Chloropropylmethyldipropoxysilane stands as a clear, colorless liquid with a slight, somewhat sweet odor. Its formula, C11H25ClO2Si, hints at a silane backbone laced with a chlorinated propyl side chain. Anyone working with this compound quickly realizes it brings a mix of flexibility and reactivity to the table, mainly because of the balance between the silane and its functional chloride group. As a chemical, it doesn’t hide — it gives off that familiar aroma found in silanes and ethers, making safe ventilation key whenever you pop open a drum or flask.
Poured into a beaker, 3-Chloropropylmethyldipropoxysilane looks like water but throws a heavier punch. Its density hovers around 0.93 grams per cubic centimeter, so it’s just shy of sinking below the water line. Splash some in an ordinary glass, and you'll spot how easily it flows—its viscosity barely slows it down. Boil it, and the whole liquid flashes off around 95-98°C at reduced pressure—a fact that matters for anyone using it in heat-intensive processes or during distillation. Don’t go near open flames: this stuff catches readily and deserves the same respect you’d give any volatile silane.
What’s most striking about 3-Chloropropylmethyldipropoxysilane is its dual reactivity. The chloride group makes it a practical starting point for further modification; it latches onto other molecules and can form carbon-silicon bonds. The two propoxy groups attached to silicon also draw attention. They hydrolyze pretty quickly in the presence of water or even ambient humidity, releasing propanol and leaving behind a reactive silanol. You won’t be waiting long for this to happen—the reaction kicks off as soon as there’s a hint of moisture.
This trait opens doors in adhesion science and in formulating high-performance coatings. A silane like this acts as a bridge between organic resins and inorganic fillers or glass fibers. Its ability to bind both oil-like and mineral surfaces comes straight from its hydrolyzable nature.
I’ve handled similar organosilicon compounds, and the lesson always repeats: keep your gloves on, use goggles, and set up real ventilation. Those propoxy groups aren’t direct toxins, but the hydrolysis byproduct, propanol, can irritate skin and eyes, and if you’re not careful, you’ll breathe it before realizing. The chloride group, once released, adds another layer—corrosiveness. So, don’t let anyone convince you that small spills don’t matter, because corrosion sneaks up on equipment and storage containers faster than you’d expect.
3-Chloropropylmethyldipropoxysilane’s niche is as a coupling agent and as a reagent for surface treatment. Glass fiber manufacturing, plastic composite enhancements, and specialty coatings all benefit from a silane like this. I’ve seen the difference it makes in composite durability and weather resistance. Yet, improper use leads to emissions and leaks; moisture in storage tanks leads to waste and sometimes accidental pressure build-up, as propanol and hydrochloric acid get produced. There’s room for better drum designs, moisture scrubbing in storage areas, and continued training for handlers. Responsible sourcing and transparent data-sharing from suppliers support safe and practical applications—something I look for in every order.
Mixing chemicals always takes a certain level of respect and understanding—one wrong combination and safety, product function, or both might fly out the window. In my own time working with silanes, I found that these materials don't always play nice with everything in the shop. Take 3-Chloropropylmethyldipropoxysilane: it stands out mainly for its reactive chloropropyl group and its dual propoxy attachments. Both factors steer the way it behaves with other materials.
First, water represents trouble right off the bat. 3-Chloropropylmethyldipropoxysilane hydrolyzes in the presence of moisture. This process kicks off much faster than many newcomers expect, especially if the workspace ambient humidity climbs above 40%. The silane reacts with moisture, releasing alcohol and forming silanols. Beyond creating unwanted byproducts, this reaction changes the chemical, often before it’s even had a chance to bond to surfaces. Factories and researchers combat this with dry conditions and carefully sealed storage. Skipping these steps wastes product and could cause sticky films in equipment lines.
Alcohols tell another story. In my experience, propanol and isopropanol make fair solvents for this silane if you need a solution for surface treatment or priming. But not all alcohols are equal; methanol might tweak the reaction kinetics, causing things to get speedy and unpredictable. Glycols and polyols have a habit of catalyzing side reactions, sometimes yielding cloudy suspensions that clog nozzles or brushes.
Reactions with amines can become sticky, both figuratively and literally. Amines tend to open up the chloropropyl side chain, starting a substitution reaction. I saw a surface-coating firm struggle for days when mixing this silane with epoxy resin loaded with amine hardeners. The mix gelled so quickly, it set in the pot, not on the part. Cool hands and trial runs become essential steps if you plan to pair these together.
On the plastics front, this silane finds a place in some vinyl, polyurethane, and even certain silicone rubbers. Still, you need to mind the additives in these plastics. Anything carrying significant water or acid will undermine bond strength. Fillers such as talc or clay usually require some extra treatment for the silane to latch on; otherwise, you wind up with weak interfaces and poor durability. Using a primer or adjusting processing temperatures sometimes bridges this gap, though it’s not a quick fix for every scenario. Surface prep often turns out to be the difference between lasting performance and a failed bond line.
Acids and bases tell the third chapter of this story. A splash of acid wakes up the hydrolysis and condensation reactions, speeding product cure but risking gelling and cloudiness. Bases chase the same path, especially the stronger hitters like sodium hydroxide or ammonia. In my early years in R&D, I watched experimental batches foam and curdle when somebody overlooked the pH of a blending tank. Team awareness and pH checks save time and inventory losses.
Learning from years on the floor, the real answer to compatibility sits with planning and rigorous testing. It means reading suppliers’ technical bulletins, running a few test mixes, and tracking results. Always anticipate byproducts and by all means, wear gloves and goggles—chlorinated silanes can irritate skin and eyes. Ventilation makes a difference, too. Factories often install dedicated exhaust systems wherever silanes show up, limiting inhalation risks and keeping the air clear for workers. Smart teams keep SDS (Safety Data Sheet) copies on hand, making sure every user knows what’s in the drum before popping the lid.
Compatibility doesn’t hinge on luck or a single mix. It’s about respecting the chemistry and maintaining good habits. Safe handling, dry storage, and an eye for surface prep save both product and effort. Constant learning, paired with real-life testing, spells the difference between success and an expensive misstep.
| Names | |
| Preferred IUPAC name | [3-(Chloropropyl)]methylbis(propoxy)silane |
| Other names |
3-Chloropropyl(methyl)dipropoxysilane 3-Chloropropylmethyldipropoxysilane 3-Chloropropyl(methyl)bis(propoxy)silane 3-Chloropropyl(methyl)dipropoxysilane 3-Chloropropyl(methyl)dipropoxy silane |
| Pronunciation | /ˌθriː-klɔːr.oʊ-ˈprəʊpɪl-ˈmɛθɪl-daɪ-prəˈpɒksi-saɪˈleɪn/ |
| Identifiers | |
| CAS Number | 2530-87-2 |
| 3D model (JSmol) | `3D model (JSmol): "CCCO[Si](C)(Cl)OCCC"` |
| Beilstein Reference | 4921668 |
| ChEBI | CHEBI:189460 |
| ChEMBL | CHEMBL1906613 |
| ChemSpider | 23130797 |
| DrugBank | DB22066 |
| ECHA InfoCard | 08e7d2cf-243d-49a2-b14e-4d8117e5a5c1 |
| EC Number | 4128-09-8 |
| Gmelin Reference | 1130873 |
| KEGG | C18607 |
| MeSH | C059159 |
| PubChem CID | 124173073 |
| RTECS number | VV9350000 |
| UNII | R36E44OZ42 |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | U033137 |
| Properties | |
| Chemical formula | C12H29ClO2Si |
| Molar mass | 270.86 g/mol |
| Appearance | Colorless transparent liquid |
| Density | 0.987 g/mL at 25 °C(lit.) |
| Solubility in water | Not miscible or difficult to mix in water |
| log P | 1.9 |
| Vapor pressure | 1.1 hPa at 20 °C |
| Refractive index (nD) | 1.4280 |
| Viscosity | 3-7 mPa.s (25°C) |
| Dipole moment | 1.24 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 386.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07, GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P273, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-1 |
| Flash point | Flash point: 93 °C |
| Autoignition temperature | 250 °C (482 °F) (DIN 51794) |
| LD50 (median dose) | LD50 (median dose): Oral (rat): > 2000 mg/kg |
| NIOSH | NA |
| PEL (Permissible) | Not established |
| Related compounds | |
| Related compounds |
Trimethylchlorosilane Dimethyldichlorosilane Methyltrichlorosilane Propyltrimethoxysilane 3-Chloropropyltrimethoxysilane 3-Chloropropyltriethoxysilane Methyldipropoxysilane Dipropoxydimethylsilane |