
Practically every time I see a chemical like 3-Chloropropyltriethoxysilane, I think about how the demand for smart surface modifications pushed its birth. In the late 20th century, research labs began seeking out organosilanes that could both bond with inorganic surfaces and leave a reactive site for later modification. Back then, glass manufacturers hunted for something to bond organic coatings to their glass products, and industries realized the significance of getting the right coupling agent. 3-Chloropropyltriethoxysilane emerged as a strong contender after years of evolving silane chemistry—first as an experimental compound, then as a commercial player as companies in the United States, Europe, and later Asia scaled up reliable synthesis. It didn’t pop up overnight, but this molecule’s ability to link silica and many polymers cemented its role in paints, adhesives, and composite materials.
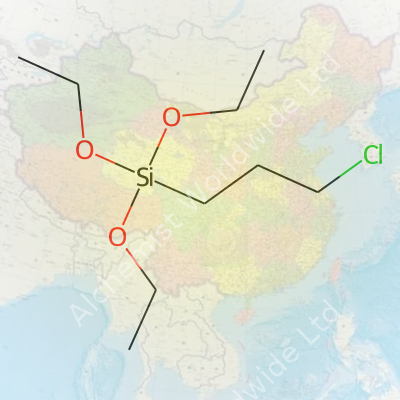
Calling 3-Chloropropyltriethoxysilane a workhorse makes sense. It’s a colorless to pale yellow liquid, defined by the structure C9H21ClO3Si. Those triethoxy groups love reacting with siliceous surfaces like glass or minerals, anchoring firmly to create organic-inorganic bridges. The chloropropyl hook that dangles from the molecule brings another layer of utility, letting users graft or swap functional groups as needed. I’ve seen companies package it in steel drums, aluminum containers, and high-density plastic bottles, ensuring it stays dry and free from contamination. Its volatility and reactivity mean you probably won’t find it sitting in glass without careful storage, and users always keep quality certificates on hand for tracking purposes and regulatory needs.
3-Chloropropyltriethoxysilane typically brings a faint, slightly sweet odor—though breathing it in is not recommended. The density lands around 1.01 to 1.03 g/cm³ at 20°C. It boils between 235 to 238°C, and the flash point hovers near 100°C, so it doesn’t catch fire as quickly as gasoline. It mixes easily with organic solvents such as ethanol, toluene, and chloroform. Get it near water, and hydrolysis happens in a flash, with the ethoxy groups swapping places for silanols, which then bond to inorganic surfaces. The reactivity of that chloropropyl tail can give both trouble and opportunity, depending on what you add next. Keeping the chemical dry and cool helps anyone storing it for more than a few weeks.
Certified shipments list purity above 97%, with impurities like water, ethanol, and dichloropropyl silanes kept to a minimum. Safety data sheets and container labels highlight hazard codes for skin and respiratory irritation, and the containers come with GHS pictograms. Packaging includes batch numbers, production dates, and expiration windows. Certification aligns with REACH, TSCA, and local regulations, reflecting the substance’s international reach. Technical sales reps provide nuclear magnetic resonance (NMR), gas chromatography, and infrared results upon request, making it easier for buyers to compare quality.
Manufacturers produce 3-Chloropropyltriethoxysilane using a hydrosilylation approach that connects triethoxysilane with allyl chloride under catalysis from platinum compounds. In an industrial setting, the process happens inside stainless steel reactors fitted with temperature controls and robust stirring mechanisms. Quality hinges on keeping air and moisture out, ensuring yields stay high and side-reactions don’t cut into profits. Modern plants recover solvents, capture off-gases, and use closed-system draining, following stricter environmental policies that cropped up as silane demand ramped up over the decades.
3-Chloropropyltriethoxysilane reacts at both ends. The chloropropyl part is open for nucleophilic substitution—swap in an amine or thiol, and you create new silanes for custom surfaces and specialty resins. Under acid or base catalysis, hydrolysis of each ethoxy group forms a silanol, which can bond covalently with metal oxides or glass. These properties allow downstream modifications and make the chemical attractive for companies chasing niche materials. I’ve seen research teams build layers onto glass slides using this exact process, engineering new biosensor chips or creating anti-fog surfaces for everyday products.
This substance appears under plenty of names: 3-chloropropyltriethoxysilane, gamma-chloropropyltriethoxysilane, triethoxy(3-chloropropyl)silane, and sometimes as CPTES or CPS. International Chemical Identifier codes, CAS numbers, and trade names—especially from large silane suppliers—add to the mix. Labeling differences often trip up less-experienced buyers, but veteran chemists know to check chemical structure diagrams instead of just names.
Safety requirements hit hard here. 3-Chloropropyltriethoxysilane brings risks of skin and respiratory irritation, so workers reach for nitrile gloves, chemical splash goggles, and fume hoods. Open bottles only happen in ventilated areas. Spills lead to slippery floors and vapors, so absorbents and scrubbers are always nearby. Waste disposal takes place through certified hazardous waste channels—landfilling silanes can haunt groundwater for generations. As someone who’s worked in labs, cutting corners with silane storage or personal protective equipment eventually backfires, usually with emergency eye-wash stations and frayed nerves.
You’ll find 3-Chloropropyltriethoxysilane in both mature and emerging sectors. Composite makers use it to treat glass fibers, hoping for a stronger grip between resin and fiber. Paint and coating companies look for longer-lasting adhesion and improved resistance against water and corrosion. Rubber and plastic manufacturers rely on the surface modification abilities to build better seals and mechanical parts. Pharmaceutical techs use it in solid-phase synthesis, and biotech groups craft reactive surfaces for diagnostics, cell adhesion, and sensor technology. Quite a few companies work quietly on modifying nanoparticles using this silane as a core step, changing the way materials interact with biological and environmental systems.
Research teams continue to push past standard uses by exploring custom grafting of catalytic sites, optoelectronic layers, and anti-microbial coatings. Patent filings point to rapidly growing uses—everything from water treatment membranes to medical diagnostics and next-generation battery separators. Open access journals track work that swaps out the chlorine group for more complex functionalities, adding new reactivity without abandoning the original silane backbone. In my experience, each breakthrough triggers a flood of industrial interest, and companies fund syndicates to control proprietary reaction conditions and formulations.
Toxicologists observe acute and chronic effects tied to inhalation, ingestion, and skin exposure. Studies in rats and fish highlight organ damage and persistent bioaccumulation in groundwater runoff, prompting European and American authorities to demand tighter handling rules. Professionals run in vitro and in vivo toxicity tests, map degradation products, and set occupational exposure limits well below 10 ppm in air. Lab incidents—burns, allergic reactions, respiratory distress—reinforce why even small leaks need fast cleanup and thorough decontamination. Recent controversy over microplastic generation in silane-derived materials has scientists reevaluating environmental release policies yet again.
Looking ahead, the push for greener, more sustainable chemistry will challenge silane manufacturers to reduce emissions and produce less hazardous waste. Researchers predict a boom in hybrid nanomaterials, medical diagnostics, and anti-microbial treatments, all thanks to the customizable nature of organosilanes like 3-Chloropropyltriethoxysilane. Companies race to develop safer alternatives, but so far, nothing matches this chemical’s combination of cost, reliability, and performance. Investment in closed-cycle synthesis, reusability, and next-gen environmental controls will decide who stays competitive as regulators in Europe, North America, and Asia lay down stricter rules. Based on my time following advances in material science, those able to marry safety, function, and sustainability will lead this industry for years to come.
On the surface, 3-Chloropropyltriethoxysilane sounds like a complex industrial chemical. Dig in, though, and you find a material with a surprisingly wide impact on daily life. It serves as a bridge between different types of materials—something I’ve noticed a lot of manufacturers struggle with when building things that need to last. In a world going heavy on composites and blends, this one molecule solves problems that would otherwise leave a lot more products falling apart quickly.
I’ve talked to folks working with fiberglass and plastics who swear by this silane for boosting how well things stick together. Using regular glue on glass fibers and plastic resin tends to fail in harsh conditions—moisture or temperature swings make everything pop off. 3-Chloropropyltriethoxysilane forms real chemical bonds at the interface, giving the finished product more durability and a better shot at holding up under stress. The chemistry gives you strong adhesion, not just a sticky surface. This cleaner, molecular-level bond means fewer warranty claims and fewer returns for manufacturers.
If you've ever dealt with electrical components, especially in humid environments, you know how critical insulation is. The silane improves insulation properties in wires and circuit boards by helping the insulating layer bond better to the core material. That’s not just technical detail; it means fewer shorts, less risk of equipment failure, and better protection for both users and expensive electronics. The safety knock-on effects from stronger insulation end up mattering in consumer electronics, cars, and even renewable energy infrastructure like wind turbines.
Painters talk a lot about paints not sticking to tricky surfaces. By treating glass, ceramics, or even some metals with a silane solution, coatings cling better and resist peeling. You can see these additives at work in scratch-resistant coatings, anti-fog sprays for glass, and more advanced automotive or marine paints. Folks who care about preventing rust or making surfaces easier to clean often rely—knowingly or not—on silane-based chemistry.
I’ve watched contractors carefully mix silane-modified adhesives to seal bathrooms, kitchens, and windows. In these wet, variable settings, regular silicone or polyurethane compounds alone tend to let go over time. Adding 3-Chloropropyltriethoxysilane gives adhesives an edge, making sure seams stay tight, and mold or water damage gets stopped in its tracks. For construction and home repair, those are real-world improvements that keep costs and headaches down.
No industrial chemical comes without questions. Workers and environmental scientists need to keep an eye on silane use. Handling can get tricky—strong odors, irritant qualities—so proper ventilation and gear are a must. Regulations require responsible disposal and careful manufacturing, and oversight from agencies helps catch problems before they affect consumers or the environment. The trade-off for stronger and more reliable products is best met through responsible sourcing and manufacturing transparency. Keeping communication open between manufacturers, regulators, and communities ensures the technology benefits everyone while minimizing risks.
My experience with chemicals stretches back to my days working in university labs, where common sense met the fine print of chemical safety data. 3-Chloropropyltriethoxysilane, a mouthful to say and tricky to store, teaches basic lessons about respect for chemical hazards. This compound packs enough punch to cause harm if forgotten on a shelf or exposed to the wrong environment. The need for careful storage comes up every time someone comes across a corroded valve or a faded label on a dusty drum. Recognizing risk early often saves time, injuries, and money.
This silane can react with moisture. Fumes from slow decomposition make for bad air quality. Leaks corrode metal and eat away paint. Skin contact can sting and leave lasting irritation. Flammable vapors threaten fire and explosion if they meet sparks or open flames. Multiply that by the curiosity of a warehouse worker or the neglect of a rushed shipment and you get chaos. Real stories of fires or costly product loss after improper storage highlight the point—every bottle stored correctly spares more than just headaches.
Storing chemicals like 3-Chloropropyltriethoxysilane starts by picking the right location. Keep the container in a cool, dry, well-ventilated spot. Room temperature works for most batches, but heat speeds up degradation. Humidity is the enemy. I’ve seen drums sweat in summer and labels peel off, so dry areas make a difference. Always keep the original label visible—guesswork about contents leads to danger.
Shelving should be sturdy, away from high-traffic aisles. Glass or plastic containers with tight-fitting lids stop leaks. Don’t use metal shelves directly, as silane can corrode some metals. Secondary containment—trays or bins under every container—catches spills and saves cleanup time. Sparks, open flames, or electric heaters should stay far from the area. Fumes can collect low to the ground, so ventilation at floor level helps catch what escapes.
I once saw a warehouse stack acids and silanes side by side. That’s asking for fumes and drama. Store 3-Chloropropyltriethoxysilane apart from anything with strong acids or alkalis. Avoid storing with water-based substances. Keep oxidizers and peroxides on completely different racks. Signs and clear demarcation matter—tired eyes in late shifts won’t double-check the fine print if it’s all on one crowded shelf.
Proper labeling cannot be overstated. Labels should list the compound, hazards, and handling advice in clear text. Every employee, not just chemists, needs a crash course on safety protocols. Practice emergency procedures. Know the nearest eyewash station and shower. I remember a rookie who grabbed a container without gloves—one quick rinse saved him a world of hurt. Safety training saves limbs and lives.
Regular checks spot small leaks before they trigger alarms. Chemical storage logs track shelf life—expired silane loses potency and gets risky. Don’t play roulette with old stock; follow disposal regulations to the letter. Simple steps—closing the lid tightly, checking for cracked containers, updating logs—turn what could become a crisis into another day at the shop.
Working in a lab brings a lot of satisfaction, but there’s always an edge of responsibility. Take 3-Chloropropyltriethoxysilane. I remember the first time a bottle landed on my bench — the sharp, irritating smell had me reaching for my respirator fast. Skin contact brought a burning itch that lingered for hours. Inhaling fumes even in low concentration set off throbbing headaches. This is no mild-mannered chemical. Its reactivity makes it valuable in manufacturing adhesives, coatings, and even glass treatments, but it also turns minor carelessness into big trouble.
The compound can damage eyes, skin, and lungs. That means a splash, or an accidental spill, doesn’t just end the workday — it can cause injuries that leave a lasting mark. Many underestimate just how quickly the liquid soaks through personal clothing. Even a small leak inside a glove can mean trouble. When I helped train interns, the lesson always circled back to this: don’t rush and never skip personal protection.
So what works? Start with personal protective equipment. Always reach for chemical goggles and a full-face shield. Regular glasses do nothing to stop this stuff from seeping in. But more than the right eyewear, choose nitrile or butyl gloves over latex. 3-Chloropropyltriethoxysilane chews through some materials far too quickly. Disposable coveralls, along with a chemical-resistant apron, save your skin and everyday clothes from absorbing fumes.
Ventilation takes the next spot. Never open containers without a fume hood running or at least strong local exhaust. Toxic vapors slip across the workspace faster than most realize. Working in a crowded lab? Post clear warning signs, and keep emergency eyewash and showers within steps, not a hallway away. Remember: seconds count.
Secure storage plays a big role in stopping problems before they start. Keep containers tightly sealed, upright, and locked in a cool area away from anything that could spark a reaction — especially acids, oxidizers, or sources of moisture. Water sets off dangerous hydrolysis that makes the air even riskier. I once saw a spill react with a wet benchtop, filling the lab with choking fumes. It was a lesson that stuck, long after we fixed the mess.
Having the right spill kit, with neutralizers and absorbent material, means nobody scrambles looking for help when accidents spill over. Training doesn’t end with reading a manual. Walk through drills every few months. Make sure everyone knows who calls for help, which doors open to safe exits, and how to isolate the affected area.
Culture eats rules for breakfast. Supervisors who set the standard — who keep up with the latest data sheets, encourage open reporting of small mistakes, or invest in new safety features — build safer environments. This does more than prevent fines or lost time. It prevents anxiety. New workers watch what the veterans do, not just what they say. Admitting when something goes wrong, sharing what happened, and updating protocol together moves safety from policy into practice.
Stay sharp with fresh Safety Data Sheets and new findings. Changes in chemical suppliers, shifts in workplace layouts, or new research can all change best practices. Keep the conversation open. The more you know, the less risk you carry out of the lab.
3-Chloropropyltriethoxysilane doesn’t invite much attention at first glance. In the lab and on the factory floor, folks crack open these drums expecting stability, often following broad claims from suppliers or tracing the vague “use within two years” printed somewhere small on the package. From my time working with organosilanes and seeing both pristine and neglected storage conditions, I’ve learned that shelf life goes way beyond a simple number.
The stated two-year promise comes from manufacturers who test under clean, ideal setups: cool, dark rooms, tightly sealed bottles, no air sneaking in. In the field, things often drift. Someone forgets to reseal a drum after a late night at the reactor. Summer heat pushes storeroom temperatures over 30°C. Moisture from a humid coastal climate creeps in, triggering slow hydrolysis. The result: the clear, low-viscosity liquid might turn cloudy, produce a faint odor, or thicken up. Each of these is bad news for downstream reactions. Trust me, nothing ruins a busy production week like a batch that gels mid-way through surface treatment—money wasted and trust with clients on edge.
3-Chloropropyltriethoxysilane acts as a bridge in adhesives, sealants, coatings, and surface treatments. Reliability rests on its ability to couple and modify surfaces properly. Hydrolyzed or polymerized silane won’t make the sort of bond you expect; your coating peels, the adhesive’s clinging power drops, or your product fails durability testing. In my experience, this story isn’t rare. A well-meaning operator uses six-month-old silane left from last quarter, unaware it already’s half gone in quality. The project fails and nobody thinks to check the age or storage history of the silane. The learning curve here can get long—and expensive.
The chemical makes its way around by drum or IBC tote. Opening a container starts a countdown: humidity from the air rushes in, and every extra day open nudges more degradation. In spaces where turnover isn’t fast, half-used containers accumulate. Vendors often recommend nitrogen-blanketing for storage, and every deviation—like those half-finished drums capped instead of purged—cuts effective shelf life from two years to as little as six months. Moisture doesn’t care about inventory plans.
The base shelf life for unopened, cool-stored 3-Chloropropyltriethoxysilane runs about 24 months from the date of manufacture, sometimes a little less. This drops fast at high temperatures. Hydrolysis forms oligomers early and can set off residue or gunk that’s hard to clean. A study from Shin-Etsu notes quality degrading within 12 months at 35°C, and Dow’s technical bulletin calls for storage under 30°C and tightly sealed conditions.
On site, I’ve seen best results by installing small hygrometers in chemical storerooms and checking seals after every use. Purchasing smaller container sizes helps keep inventory fresher. Training matters too; staff remember which products demand extra care after seeing a ruined batch or hearing about a failed inspection.
For those running a busy blending or treatment operation, set up clear routines. Record opening dates. Inspect for cloudiness or odd smells before every use. Move older stock up the queue and flag anything over one year for testing before application. If quality holds, great; if not, you’ve dodged a much bigger headache. Shelf life never stays a set number—good habits and honest checks protect production and reputation.
In the practical world of chemicals, 3-Chloropropyltriethoxysilane does a steady job. It sits on shelves across labs and factories because it brings a useful mix of a chlorinated propyl group and triethoxysilane backbone. Those who know coatings, sealants, or resilient composites often ask how well it gets along with other silanes and polymers. I’ve watched a team get excited about it in a formulation meeting, trying to solve something as tangible as squeaky automotive trim.
People often pair this silane with aminosilanes, vinylsilanes, or epoxy-functional silanes. The goal is always to improve adhesion or weather better. The triethoxy groups provide anchor points on substrates — glass, metal, or minerals. That leaves the chloropropyl part ready to bond further. Cross-linking reactions count on water or simple humidity to get started, so there’s no need for special tricks in most shop-floor environments.
Chemists sometimes run into roadblocks with hydrolysis rates or competing functional groups. Chlorine can make certain mixes less forgiving, introducing a risk of byproduct formation. Careful dosing and sequence keep things predictable. Combining with aminosilanes, for instance, usually clicks due to complementary reactivity. My friend once tried a 50:50 blend and found the glass fiber surface held up better in aging tests. Facts like this show the approach isn’t just theory; it’s how products survive the real world.
People rarely use 3-Chloropropyltriethoxysilane on its own. In sealants and paints, you find it as a coupling agent. It binds fillers to resins, boosting both durability and water resistance. The ethoxy groups bond with minerals; the chloropropyl segment holds on to the organic resin’s backbone. In polyurethanes, it sometimes speeds up curing or tee’s up cross-linking, reinforcing the final product.
Issues can pop up. The reactive chlorine can tangle with strong nucleophiles in resin mixes, sometimes creating unwanted side products. Over time, a team learns to avoid certain hardeners or tailor polymer chain lengths. I’ve seen this managed with small pre-blend tests instead of large-batch guessing. In acrylic or epoxy systems, pilot projects often reveal the right ratio and reaction time.
Using chemicals like this means paying close attention to safety and compliance. The chlorine atom draws regulatory scrutiny, as do the vapors from the ethoxy groups. Most suppliers offer solid safety data sheets, and industry guidelines keep everyone honest. Proper storage, correct PPE, and local exhaust ventilation are basic steps, but they keep the shop running and protect health. I recall a project where a small spill set off air monitors—luckily, standard training kept everything contained.
Compatibility depends on knowing the chemistry and making tests count. Blending with other silanes often brings out the best properties, provided the team understands the details—order of mixing, pH control, and solvent choice. Mixing into polymers adds mechanical performance, but it’s never a plug-and-play move. Most success stories come from project teams who run pilot batches, talk through mistakes, and keep digging into data.Fact-based adjustment, collaboration, and a willingness to revisit earlier choices build better products and fewer surprises. That’s the core of making chemicals like 3-Chloropropyltriethoxysilane work in the field.
| Names | |
| Preferred IUPAC name | 3-chloropropyl(triethoxy)silane |
| Other names |
3-(Triethoxysilyl)-1-chloropropane 1-Chloro-3-(triethoxysilyl)propane γ-Chloropropyltriethoxysilane Triethoxy(3-chloropropyl)silane Silane, 3-chloropropyltriethoxy- |
| Pronunciation | /ˌθriː-klɔːr.əˈprəʊ.pɪl.traɪ.iːˌθɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 5089-70-3 |
| 3D model (JSmol) | `3d7a8c86878183876d9b67eb7296d21574724f474a6c64616c4e454e5c4c504149434458415c61614a4b47494b584b602e3735666269605e686f6c687444686b716e42415d16715e6170677c6e78607e247c6572736c6a76636979796749404e683063676f62584d767c626c797c636f6e763c3236c8` |
| Beilstein Reference | 1718739 |
| ChEBI | CHEBI:87344 |
| ChEMBL | CHEMBL1089951 |
| ChemSpider | 80722 |
| DrugBank | DB14271 |
| ECHA InfoCard | ECHA InfoCard: 100.019.198 |
| EC Number | 203-586-1 |
| Gmelin Reference | 1620274 |
| KEGG | C19552 |
| MeSH | D016674 |
| PubChem CID | 67436 |
| RTECS number | GV2175000 |
| UNII | 75ZI1Y1302 |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C9H21ClO3Si |
| Molar mass | 278.82 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Pungent |
| Density | 0.943 g/mL at 25 °C |
| Solubility in water | Solubility in water: reacts |
| log P | 2.9 |
| Vapor pressure | 0.4 hPa (20 °C) |
| Acidity (pKa) | 10.5 |
| Refractive index (nD) | 1.414 |
| Viscosity | 2 mPa.s (25°C) |
| Dipole moment | 1.98 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 439.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -713.65 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1966 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H226, H302, H315, H319, H332, H411 |
| Precautionary statements | Precautionary statements: P261, P280, P301+P312, P305+P351+P338, P337+P313, P308+P313 |
| NFPA 704 (fire diamond) | 2-2-1 |
| Flash point | 74.0 °C |
| Autoignition temperature | 270 °C |
| Lethal dose or concentration | LD50 Oral - rat - 1,892 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 1897 mg/kg |
| NIOSH | GV2150000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Chloropropyltriethoxysilane: Not established |
| REL (Recommended) | 10 ppm (60 mg/m3) |
| Related compounds | |
| Related compounds |
Trimethoxypropylsilane Triethoxyvinylsilane 3-Aminopropyltriethoxysilane 3-Glycidoxypropyltrimethoxysilane Methyltriethoxysilane Phenyltriethoxysilane 3-Mercaptopropyltriethoxysilane |