
Silane compounds have been part of industrial chemistry since the early 20th century, but things really kicked off for organofunctional silanes in the 1960s. Manufacturers were looking for ways to bridge the gap between inorganic fillers and organic resins, and this created a huge demand for coupling agents. 3-Glycidyloxypropylmethyldiethoxysilane climbed out of the lab and into mass production thanks to this need. Today, its name might trip you up, but it tells a story about chemistry responding to real-world problems — making plastics tougher, paints last longer, and electronics more reliable. The evolution of this compound reflects the way chemical industries shift focus, improve methods, and respond to the call for safer, cleaner materials.
3-Glycidyloxypropylmethyldiethoxysilane sits among the group of epoxy silanes, acting as a bridge between organic polymers and inorganic substances. This makes it valuable in adhesives, coatings, sealants, and composites. What stands out is its dual functionality — it has both epoxy and silane groups. Epoxies stick tightly to organic surfaces, while silanes anchor to materials like glass, minerals, or metals. I’ve seen manufacturers use this silane to boost the bond strength in fiberglass-reinforced plastics and to give paints some serious staying power against weather and wear. Industries rely on it for performance improvements that show in the real world, not just on data sheets.
Its structure blends an epoxy ring and diethoxysilane functionality, making it a clear or slightly yellowish liquid at room temperature. The molecular weight is about 264 g/mol. Boiling point hovers around 300°C. It dissolves in organic solvents—ethyl acetate, toluene, alcohols—and it can hydrolyze in water, forming silanols that bond to surfaces. That transformation is crucial: once it hits moisture, the siloxane bonds form, and this locks everything in place. It’s got a modest vapor pressure, so handling in open containers calls for some care. You find a mild, ether-like odor when opening a fresh bottle, and it flows easily, integrating into mixtures without stubborn clumping or settling.
Industry standards set high purity for this chemical — typically upwards of 98%. The CAS number is 2897-60-1, and packaging requires careful attention, often in amber glass or HDPE drums to keep light and air out. Labels carry hazard statements tied to its flammability, skin and eye irritation, and environmental care. I’ve seen strict batch tracking, as the performance of end-products—coatings, adhesives, composite resins—can depend on the tiniest impurities in raw silanes. Some suppliers add stabilizers or inhibitors to slow down hydrolysis, and these are always clearly marked in the technical datasheet. Accurate documentation makes life simpler for workers and emergency responders alike.
Manufacturers synthesize 3-Glycidyloxypropylmethyldiethoxysilane starting with reaction between glycidol and methyldiethoxysilane using a catalyst under mild heating. This process draws on principles found in organosilicon chemistry, where protecting sensitive groups from premature reactions shapes the entire workflow. Years ago, batch reactions ruled, but now continuous flow methods dominate for tighter control of product quality and lower waste. Water used in the process gets careful treatment to neutralize any reactive offshoots. I’ve seen research push for greener methods, using less toxic catalysts and recycling solvent streams to cut the environmental footprint.
The epoxy ring in this molecule opens under acidic or basic conditions, making it reactive for crosslinking with amines, anhydrides, and even carboxylic acids. Meanwhile, diethoxysilane groups hydrolyze in the presence of moisture, forming silanol groups that condense onto glass, metal oxides, and other substrates. This handy ability to “glue” organic and inorganic bits together underpins much of what makes this silane so useful. People have played with its backbone, adding different organofunctional groups or using it in tandem with other silanes to tune product properties. New derivatives keep emerging, tailored for electronics, surface treatments, and polymer chemistry.
Marketed under many names, this compound appears as GLYMO silane, KH-560, A-187, and several supplier-labeled abbreviations. Chemists may call it Epoxy silane, 3-(2,3-epoxypropoxy)propylmethyldiethoxysilane, or methyldiethoxy(3-glycidyloxypropyl)silane. These variations point to the same core molecule, but trace additives or stabilizers set each commercial product apart. The number of labels sometimes causes confusion, especially when global supply chains stretch from North America to Europe and Asia. In the lab, a quick check of CAS number and technical grade ensures you’re working with what you expect.
Handling this chemical needs attention to detail. It irritates skin, eyes, and mucous membranes, and can react with water or humidity in the air to form hazardous byproducts. In my time around silane processing lines, gloves, goggles, and sturdy ventilation systems are always non-negotiable. Emergency showers and spill kits stand ready in production facilities. Storage containers stay sealed tight, often under inert gas like nitrogen, to prevent accidental hydrolysis or fire. Waste streams go for neutralization before disposal, meeting local environmental regulations. Industry bodies like OSHA and REACH keep updating guidelines, reflecting new research on worker health and broader risks.
Manufacturers put 3-Glycidyloxypropylmethyldiethoxysilane to work in adhesives, sealants, composites, and coatings. Its real-world value shows up in fiberglass parts, electronic encapsulants, industrial paints, and even dental materials. Epoxy composites get stronger, resist water, and keep their shape under tough conditions. In paints, the silane helps pigment stick to surfaces, making finishes tougher and less likely to peel or fade. In electronics, it promotes insulation and protects sensitive circuits from humidity. Product designers rely on this silane to meet the push from consumers for longer-lasting, higher-performing goods.
New needs drive silane research, and this molecule sits right in the middle of the action. I’ve seen academic labs dig into ways to reduce processing temperature, use bio-based solvents, and engineer new silane hybrids for nano-coatings. Researchers want to curb VOC emissions and cut toxic byproducts in adhesives that end up in packaging, building, and electronics. Material scientists share breakthroughs at conferences, showing off new blends that use less silane but deliver stronger, cleaner results. Open sharing of findings across business and academic lines speeds up improvement, and digital tools now let teams model reaction safety and predict material performance before the first small batch leaves the flask.
Safety studies show that, like many epoxy and silane compounds, this chemical can trigger skin and respiratory irritation. Long-term animal studies look for chronic effects, with regulatory agencies asking hard questions about inhalation and dermal contact. Studies so far point to low acute toxicity but urge respect for repeated exposure risks, especially in workplaces without strong protective measures. Wastewater containing this silane requires treatment, as the breakdown products can stress aquatic systems. The science points out gaps—there’s still work to be done on understanding its behavior in real industrial settings and the environment at large. I’ve seen calls for better transparency on chemical safety data from makers and users, recognizing public concern about synthetic chemicals in daily life.
The road ahead for 3-Glycidyloxypropylmethyldiethoxysilane looks shaped by sustainability and tightening safety benchmarks. Companies hope to boost recyclability in composites, find less toxic alternatives for hazardous processes, and cascade greener chemistry through supply chains. Demand for lightweight, durable goods in transport and electronics won’t slow down, and this molecule’s versatility keeps it in the toolkit. Digital monitoring, better worker safety protocols, and cross-border chemical regulations will shape its footprint. Scientists and engineers work toward silanes that match high performance with low hazard, giving industry and consumers new choices without compromise.
3-Glycidyloxypropylmethyldiethoxysilane, sometimes a mouthful to say, shows up behind the scenes in products we touch every day. Its long name hides its tough job — acting as a bridge where things usually have trouble sticking together. Factories lean on it to link materials that wouldn’t otherwise cooperate, like glass and plastic, or metal and resin. If you’ve ever seen a composite material, a wind turbine blade, or the neat bond holding the sole of your running shoe, odds are you’ve seen its work, just without knowing.
Take adhesives. Cheap superglue dries out or slips if you try sticking tough stuff. That’s where this silane steps in. Its molecules weave themselves right into the surfaces, helping create a grip that doesn’t let up. In my own garage, I remember patching together some old fiberglass and wondering why the repair kit came with a weird-smelling “primer.” Turned out, that primer carried a silane compound, boosting the glue’s power for years instead of weeks.
Paint makers turn to it, too. Coatings with a dose of this compound form better shields against water and scrapes. Window manufacturers use it so glass won’t fog as quickly or gather dirt as much. Electronics companies turn to silane chemicals during chip manufacturing, linking silicon and plastic reliably enough to power our tablets, phones, and chargers through thousands of cycles. Lab tests back up these choices – surfaces treated with this silane beat untreated ones for peel strength, weather resistance, and lifespan.
Nobody enjoys peeling off flooring tape, only to see sticky bits left behind or worse, a weak bond that gives way after a few months. The type of silane we’re talking about transforms that problem. It keeps adhesives stuck, even through heat, moisture, or vibration. In my experience working with electronics guys, people trust it to seal screens, glue connectors, and hold together layered components because they know it won’t give up easily.
Factories value every minute of production time. Delamination or failed bonds mean scrapping parts, wasting resources, and dealing with delays. By boosting bond strength up front, companies cut down quality control problems, saving money and protecting reputations. Silane helps keep that conveyor belt moving.
On the flip side, using chemicals in bulk means responsibility. Factories handling this silane must follow careful safety procedures, since concentrated silanes can irritate skin or lungs. Workplace monitoring and solid training keep hazards in check. The smart move involves choosing lower-emission products and building tight ventilation into production lines.
Scientists keep tinkering, developing greener versions with less volatile organics. There’s a real push for safer, more environmentally friendly silane solutions. Regulatory agencies conduct regular reviews, and responsible manufacturers tweak their processes for lower waste and greater employee safety, aiming for industry certifications that prove their commitment.
This unwieldy-named chemical quietly pushes industries forward. Whether you’re weatherproofing your deck, installing solar panels, or tapping away on your phone, chances are high that its invisible hand holds those materials together. The more we learn about it, the more important it becomes to balance performance, safety, and responsibility, keeping innovation moving while safeguarding people and the planet.
Any project aiming for stable, long-lasting performance in tough environments relies on chemical bonds that don’t give up easily. 3-Glycidyloxypropylmethyldiethoxysilane, or GPMDES for short, is built for that job. It bridges the world between organic materials like plastics and resins and inorganic substances like glass, stone, or metals. Having worked in labs experimenting with adhesives and coatings, it’s clear that the main benefit comes from the unique way this molecule behaves at interfaces. Its structure includes both silane and epoxide groups. This means it sticks itself to glassy or metallic surfaces using the silane end, while the epoxy-end grabs onto organic resins like epoxies and polyurethanes. This dual nature means composites don’t fall apart when weather, humidity, or heat come into play.
Anyone who has repaired broken ceramics or tried to coat metal parts knows how water finds its way in, causing weak spots or corrosion. GPMDES doesn’t just block moisture; it reacts with surface water on glass or metals, locking itself in as a tight chemical bond. This reaction makes surfaces less likely to peel or degrade. In automotive and construction work, such durability keeps replacement costs and maintenance low. For manufacturers, it means fewer callbacks and wasted inventory. In fact, studies show that treated glass fiber composites hold up better in truck panels and bridges exposed to rain and road salt.
A single bottle of GPMDES covers a lot of ground. It fits right into systems with epoxy, phenolic, or even acrylic resins. I’ve seen it used in marine paints, wind turbine blades, and even electronics encapsulation. It doesn’t just get along with one or two formulas—its structure weaves into many polymeric webs, which makes it valuable for product designers chasing reliability. It helps push paint and sealant formulations beyond existing limits. Fewer failures show up under thermal cycling, and repairs stick the first time.
No specialty chemical is risk-free. In the lab, personal protective equipment isn’t just for show. GPMDES can irritate the skin or eyes, and improper inhalation can lead to longer-term issues. Safety Data Sheets insist on goggles and gloves. Following these rules gets results without accidents. Poor storage—especially leaving bottles open on the bench—leads to degradation; the silane group draws water from the air, which weakens its performance. Smart handling—like capping bottles and choosing dry, temperature-controlled storage—preserves its value.
People focus more on sustainability, and GPMDES finds a place here. Stronger composite bonds mean lighter cars, less fuel, and longer-lasting infrastructure. While manufacturing any chemical comes with an environmental footprint, making materials that survive decades instead of years balances that impact. Research continues on safer, less wasteful production methods with this silane. Regulatory agencies encourage limiting emissions during use—and tracking exposure among workers and end users.
From my own projects fixing boats or building weatherproof electrical boxes, dependable bonding makes all the difference. 3-Glycidyloxypropylmethyldiethoxysilane gives users one of the strongest connections possible between otherwise incompatible worlds. This earns its place in the toolkit of anyone building for the long haul.
Anyone who’s ever worked with 3-Glycidyloxypropylmethyldiethoxysilane knows how important it is to respect chemicals like this. A mouthful of a name, and just as complex in behavior, this substance isn’t found in most household garages or average workshops. It turns up in specialized manufacturing, coatings, or adhesives labs, where folks know a bit about chemical safety.
I’ve handled epoxies, silanes, and a whole lot of substances that don’t forgive sloppy habits. This compound isn’t outright explosive or legendary for violence, but it has a nasty side. Direct contact can cause irritation. If it spills or vaporizes, it turns air and skin into risk factors. A splash in the eye, accidental inhalation, or absent-minded cross-contamination during cleanup can bring trouble.
Storing this silane safely starts with controlling its friends: moisture and heat. Let even a little water sneak into the drum and you’ve got a chemical stew that starts to react before you want it to. I’ve seen labels peel or containers swell from reactions that came out of the blue. This means dry, well-ventilated rooms with good temperature control matter more than a spot on a random shelf.
Dedicated chemical storage spaces give the best peace of mind. Use sealed containers made to lock out both air and water. Keep them off the ground, away from heat sources, sunlight, or any process that might shake things up unexpectedly. Silane will search for chances to break down if stored poorly.
When handling this material, personal protection tops the list. I rarely pick up any reactive chemical without gloves, goggles, and a proper coat. With 3-Glycidyloxypropylmethyldiethoxysilane, this has to be standard. Nitrile gloves help block out both the liquid and possible vapor. Chemical splash goggles or a face shield work better than eyeglasses.
Good ventilation makes a difference. Through the years, I’ve seen how working in cramped or stuffy spaces lets vapor settle, raising health risks. Fume hoods and exhaust fans keep air moving, removing much of the trouble before it finds you.
Spills or drops do happen. No matter how careful you are, a lid slips or a bottle gets bumped. Always have suitable absorbents close by—sand or commercial neutralizing agents, not paper towels that soak and spread. Cleaning up isn’t complete until surfaces are washed down and hands scrubbed, no matter how small the mess looked.
One mistake I’ve seen in many workplaces is casual documentation. Safety data sheets often end up unread, hiding in a file. Regular training, clear labeling, and up-to-date records help everyone—old hands and new faces alike—understand both risks and responses. Emergency showers and eyewash stations within easy reach aren’t optional. Quick reaction can make all the difference in an accident.
Reducing risks starts with careful planning. I always press for smaller stock quantities if the process allows. Smaller containers mean less waste, less exposure, and easier containment. For large-scale users, dedicated staff keep a tighter watch on procedures, reducing mistakes from divided attention. Internal audits and peer checks build habits that turn safety into reflex, not routine.
Every chemical brings lessons, especially ones as unforgiving as silanes. Being prepared, respecting the material, and never rushing lead to a safer lab or factory, every single time.
Ask anyone familiar with manufacturing or adhesives about silane compounds, and 3-Glycidyloxypropylmethyldiethoxysilane comes up as a key ingredient. This tongue-twister of a molecule often finds its way into the coatings, sealants, and plastics that shape modern life. It does its job well, binding surfaces or boosting the flexibility of cured products, but most people don’t give a second thought to what happens long before it gets buried inside a composite.
I’ve stood next to workers handling chemicals like this in tight, stuffy spaces. Most had only goggles and gloves. Direct contact with 3-Glycidyloxypropylmethyldiethoxysilane causes irritation—skin gets red and itchy, and a splash in the eyes stings like nothing else. Inhaling vapors or dust sends a sharp sensation deep in the nose and throat. Reports from industrial hygiene studies back up these observations, noting that the eyes, skin, and lungs are the main targets. Many epoxy-based silane compounds trigger allergies over time, which means a single bad exposure can make future reactions much worse.
Some chemicals seem harmless based on short-term use, but silane chemistry often hides long-term risks. Animal testing has shown that swallowing large amounts of this type of silane can impact organ systems, especially the liver and kidneys. Extended vapor exposure, even at low concentrations, can inflame lung tissue. More important, the “glycidyl” group in the molecule falls into a class of epoxy-related chemicals under close review for carcinogenic potential. The World Health Organization flagged analogous compounds for their links to DNA damage, nudging workplaces to pay attention.
Despite wide use, everyday folks don’t see warning labels on finished products. Most exposure happens in factories or small shops mixing epoxy or sealant, but disposal practices matter just as much. If a drum leaks, groundwater can become a silent carrier. Watching city recycling centers sort through paint and chemical shipments shows there’s confusion around safe handling, even among trained workers. The EPA and OSHA have provided some guidelines, but the science keeps shifting as we collect better long-term health data.
Chemical safety never comes from trust alone. Relying on a safety data sheet tucked away on a clipboard won't help if staff rush through jobs without training. In my experience, regular hazard training, simple warning posters, and proper ventilation matter more than fancy equipment or expensive filters. Mechanical ventilation—good, strong airflow through open doors and roof vents—cuts down on vapors where people actually stand and work. Quick rinses in emergency showers or eyewash stations save eyesight and skin, provided they’re easy to reach.
Switching to less hazardous alternatives where possible or limiting the use of open mixing helps. Companies that do it well build a culture where people call out problems and fix them without fear of delay or cost. Regulators play a role too, but it’s those small, everyday choices that actually keep folks safe. For anyone working with silane compounds, treating them with healthy respect and a few practical measures can prevent years of trouble.
Anyone who’s patched up a fiberglass canoe knows all too well that the right resin changes everything. In composites, 3-Glycidyloxypropylmethyldiethoxysilane (let’s call it GLYMO for short) steps up as a bridge between the glass fibers and the epoxy resin, making sure the final product doesn’t just stick but stays reliable over time. Big names in automotive and aerospace lean on these properties to churn out lightweight, rattle-free panels and components that shrug off moisture and heat. I remember working on a project where switching to a silane-modified resin translated to fewer warranty claims and happier assembly-line supervisors.
Sealants take a beating outdoors, facing everything from hail to city grime. GLYMO steps up as a treatment for glass, concrete, and ceramics. By anchoring itself to the surface and bonding with coatings or paints, it increases resistance to peeling and fading. Coating manufacturers add GLYMO to formulas aiming for longer service on office towers, stadiums, or subway stations. Staying ahead of repair cycles saves money, but it also cuts waste—a fact facilities managers notice in their budgets. Working construction sites made it clear: when you don’t have to recoat every other year, schedules open up, and everyone’s less stressed.
If there’s one place where reliability never gets old, it’s electronics. Printed circuit boards, semiconductors, and sensors need moisture protection, especially with today’s push for wearable tech and smart gadgets. GLYMO finds a home in encapsulants and potting materials because it improves electrical insulation and ensures tiny connections stay protected from damp air and dust. This matters to the average person just trying to get more than a year out of a phone or fitness tracker. Having been in repair shops, I’ve seen how better encapsulation means fewer early device deaths. Customers notice one less thing to worry about.
Medical technology can’t cut corners. Flexible catheters, implanted sensors, and diagnostic chips all benefit from the stable, moisture-resistant properties that GLYMO delivers when blended into silicone rubber or polyurethane coatings. Hospitals depend on tubing that doesn't crack or harbor bacteria, and patients want implants that last. I’ve seen the anxiety in families waiting for test results or surgery, knowing that better materials mean fewer complications later. Published clinical device reports point to longer, safer service life with silane-modified materials—in the end, it’s about trust in the hands of clinicians and patients.
GLYMO plays a part in pushing industries toward tougher, more reliable, and eco-friendly goods. There’s also pressure to keep its chemistry safe for both workers and the environment. Companies that develop training and protective equipment for those who handle these chemicals lower risks for everyone. Regulatory agencies push for safer production methods, too, tightening up on volatile substances. As demand for sustainable tech rises, labs are exploring plant-derived silanes to lighten the environmental load. Staying transparent with safety data and adopting greener options keeps both manufacturers and end users in the loop and ahead of the curve.
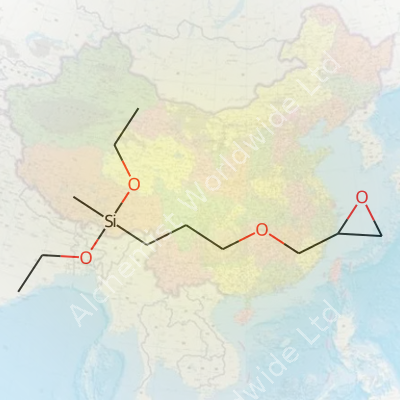

| Names | |
| Preferred IUPAC name | 3-Glycidyloxypropyl(diethoxy)methylsilane |
| Other names |
3-Glycidyloxypropylmethyldiethoxysilane 3-(2,3-Epoxypropoxy)propylmethyldiethoxysilane Methyldiethoxy(3-glycidyloxypropyl)silane MEGDEP |
| Pronunciation | /ˈθriː-ɡlɪˌsɪdɪˌlɒksiˌprəʊpɪlˌmɛθɪlˌdaɪˌiːˈθɒksiˌsaɪleɪn/ |
| Identifiers | |
| CAS Number | [[2897-60-1]] |
| Beilstein Reference | 1911076 |
| ChEBI | CHEBI:82777 |
| ChEMBL | CHEMBL3222771 |
| ChemSpider | 2034271 |
| DrugBank | DB14683 |
| ECHA InfoCard | 03ad237d-7c11-4ac5-872c-5eb842f6b03c |
| EC Number | 216-893-0 |
| Gmelin Reference | 104116-30-9 |
| KEGG | C19705 |
| MeSH | D001818 |
| PubChem CID | 86656 |
| RTECS number | VV5950000 |
| UNII | ETJ7ZBG4E6 |
| UN number | UN3334 |
| Properties | |
| Chemical formula | C11H24O5Si |
| Molar mass | 262.41 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Characteristic |
| Density | 1.06 g/mL at 25 °C (lit.) |
| Solubility in water | Soluble |
| log P | 0.6 |
| Vapor pressure | 0.1 mmHg (20 °C) |
| Acidity (pKa) | pKa ≈ 16 (epoxy group), silane not ionizable |
| Basicity (pKb) | 6.5 |
| Refractive index (nD) | 1.4270 |
| Viscosity | 15-25 mPa·s |
| Dipole moment | 4.1617 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 621.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause an allergic skin reaction. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 108°C |
| Autoignition temperature | 310°C |
| Lethal dose or concentration | LD50 Oral Rat 8025 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 2407 mg/kg (rat, oral) |
| NIOSH | GV2465000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 mg/m³ |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
3-Glycidyloxypropyltrimethoxysilane 3-Glycidyloxypropyltriethoxysilane 3-(2,3-Epoxypropoxy)propyltrimethoxysilane 3-(2,3-Epoxypropoxy)propylmethyldimethoxysilane |