
3-Isocyanatopropyltriethoxysilane emerged from the intersection of two growing industries: silicon-based chemistry and the need for high-performance adhesion promoters. Back in the twentieth century, chemists started piecing together organic and inorganic worlds, trying to marry flexible carbon frameworks with the stability of silicon-oxygen bonds. Researchers recognized early on that introducing isocyanate groups into silane molecules brought massive advantages, particularly in coatings and sealants. By coupling silanes with isocyanate functions, the resulting compounds could reach deeper into both mineral substrates and organic polymers, setting a new benchmark for coupling agents. The development of 3-Isocyanatopropyltriethoxysilane followed a demand for advanced composites that could withstand the elements while holding on to their structural integrity, especially in automotive and construction fields.
In daily chemical use, 3-Isocyanatopropyltriethoxysilane plays the role of molecular go-between. It latches onto inorganic surfaces, like glass, metal, or stone, with its trio of ethoxy pistons. Its isocyanate head reaches out to organic matrices, reacting with active hydrogen atoms and making a strong, almost stubborn bond. Its effectiveness stands out in laminates, adhesives, and even electronics encapsulation. Compared with other silane coupling agents, 3-Isocyanatopropyltriethoxysilane sets a standard for chemical versatility. Factories churn out this compound for use in making anything from high-bond automotive parts to water-resistant seals in industrial flooring.
This compound appears as a clear to yellowish liquid, with a characteristic pungent odor that comes from the isocyanate group. With a molecular formula of C10H21NO4Si, its molecular weight hovers at about 247 g/mol. The isocyanate function imparts a level of reactivity that demands respect and caution. It usually exhibits a boiling point close to 255°C and a density measured around 1.02 g/cm³ at room temperature. It doesn’t dissolve in water, but it reacts steadily with moisture, releasing carbon dioxide and forming urea linkages. This hydrolytic behavior calls for moisture-controlled environments during storage and use. The ethoxy groups, on the other hand, ensure compatibility with a wide variety of siliceous materials, opening doors for surface tailoring across industrial domains.
Pure, top-quality 3-Isocyanatopropyltriethoxysilane generally comes with a minimum assay of 97%. Impurities, such as traces of free amines or silanols, have strict upper limits because subpar contaminants may wreck process reliability or trigger side reactions. Containers bear all the necessary hazard warnings: corrosive, sensitizer, and toxic upon contact or inhalation. Material safety data sheets urge gloves, goggles, and strict fume control during handling. Global harmonization standards call for serial labeling of batch numbers, origin, date of manufacture, and CAS number (24801-88-5) for full traceability and compliance with international trade.
Industrial synthesis usually starts with 3-aminopropyltriethoxysilane, which reacts with phosgene or an alternative carbonylating agent such as triphosgene. The process brings challenges because phosgene is toxic and needs closed-loop systems to keep workers safe. After the replacement of amine’s hydrogen by a carbonyl group, post-reaction purification removes excess phosgene, unreacted amines, and hydrochloric acid. Distillation under reduced pressure ensures minimal decomposition and maximum recovery. Leading chemical companies have adopted greener phosgenation technologies and found ways to recycle solvent streams, cutting down both risk and waste footprints.
The compound puts its isocyanate group front and center in any reaction. That group finds any available NH, OH, or SH group and forms a new urethane, urea, or thiocarbamate bond. The silane side takes action during surface treatment of glass fibers, ceramics, or metal oxides. Upon contact with a trace of water, the triethoxysilane groups hydrolyze into silanols, which then condense with –OH groups on the substrate, producing robust Si–O–Si bonds. If chemists want to tweak the reactivity further, they can swap alkoxy groups or cap the isocyanate for delayed crosslinking. This flexibility makes it crucial for custom-tailored adhesives, composites, and functional coatings designed to survive everything from freeze-thaw cycles to immersion in corrosive media.
On chemical catalogues and industrial inventories, 3-Isocyanatopropyltriethoxysilane often pops up under other names: γ-isocyanatopropyltriethoxysilane, 3-(Triethoxysilyl)propyl isocyanate, and Silane, isocyanatopropyltriethoxy-. Trade names vary across suppliers—Dynasylan® ICPTES and Silquest® A-Link NCO serve as common examples from major producers. It pays to double-check molecular structures and datasheets, especially since confusion between amino and isocyanate silanes may derail production runs or trigger costly safety incidents.
Open a drum of 3-Isocyanatopropyltriethoxysilane, and the first thing that hits is its send of danger. Isocyanates irritate skin, eyes, lungs, and can cause allergic reactions, some long-lasting. Workers must lock down strict PPE—nitrile gloves, full face shields, and chemical-resistant aprons. Engineering controls come into play: extractor fans, leak-proof delivery, and emergency wash stations next to each work zone. Regulatory agencies demand continuous monitoring of workplace atmospheres, since even trace inhalation can sensitize lungs or spark acute symptoms. I remember seeing first-timers struggle with unexpected coughs or rashes, so training never goes out of date. Keeping air dry during handling prevents hazardous hydrolysis and fume buildup. Emergency drills, spill kits, and neutralizers stand ready, because no one wants to see what happens when moisture hits a puddle of this stuff.
3-Isocyanatopropyltriethoxysilane plugs key performance gaps across manufacturing. In composite materials, it acts like a double-agent, binding glass fibers to organic resin, beefing up both flexural and impact strength. Automotive manufacturers lean on this silane for lightweight, sturdy bumpers and dashboards that put up with wear, sunlight, and road salt. Sealant producers blend it into silicone and polyurethane formulas, seeking long service lives even at temperature extremes or in wet climates. Electronics factories look to it in the race toward miniaturization—better coupling between encapsulating gels and fragile silicon chips prevents delamination under constant thermal cycling. On construction sites, specialty paints, grouts, and insulation panels see markedly less cracking or surface peeling after silane treatment. This chemical’s crosslinking power has made serious advances in renewable energy too, from windmill blades to corrosion-resistant coatings on lithium battery cases.
Ongoing research centers on the kinetics of hydrolysis and the lifespan of the siloxane bonds. Lab teams harness spectroscopy and electron microscopy to understand how isocyanate-modified silanes migrate, anchor, and organize themselves on mineral surfaces or within polymers. Performance testing never ends, whether it’s 10,000-cycle flexing, salt spray exposure, or humidity aging. Green chemistry has taken over as a guiding principle. Researchers look for new synthetic routes to cut down on phosgene use, lower emissions, and improve the atom economy. Teams are unraveling how molecular tweaks alter bonding behavior, mechanical properties, or electrical resistance, making it possible to custom-fit new applications. Industry-academic collaborations yield rapid improvements, and tech transfer offices keep a close eye on intellectual property in this competitive chemical sector.
Toxicologists have known about the hazards of isocyanates for decades. Laboratory animal tests show that both acute and chronic exposure can lead to respiratory inflammation, eye damage, and sometimes long-term sensitization that triggers asthma on repeat encounters. Human case studies, mostly from industrial accidents or insufficient ventilation, demonstrate similar health impacts. Regulatory thresholds continue to tighten, especially in Europe and North America. Material safety sheets require full disclosure of all hazards and suggest robust first aid protocols in case of spills, inhalation, or skin contact. The chemical’s environmental toxicity remains under close study, with scientists scrutinizing breakdown products and their persistence in groundwater or soil. Manufacturing plants adopt stricter waste treatment and air filtration systems, not only to comply with evolving legal standards, but also to protect neighboring ecosystems.
As new challenges in sustainability and performance take hold, 3-Isocyanatopropyltriethoxysilane stands at a crossroads of tradition and innovation. Demand for more durable, lighter-weight materials—whether in green building, alternative transportation, or electronic devices—keeps pushing the limits of what functional silanes can deliver. Multinational firms devote resources to next-generation derivatives, tweaking substituents or coupling agents to provide even tighter control over cure rates, water resistance, and eco-friendliness. Regulatory environments push for greater transparency and greener production, prompting firms to develop low-emission processes and closed-loop recycling for silane-based composites. My experience observing how industrial needs shift over time makes me believe that the role of intelligent coupling agents, especially those bridging organic and inorganic worlds, isn't going anywhere. Chemical engineers and scientists will keep unlocking new uses for this versatile molecule, provided that health, safety, and environmental concerns stay front and center.
Walk into a lab or manufacturing site that deals with coatings, adhesives or composites, and odds are good you’ll run across a bottle labeled 3-Isocyanatopropyltriethoxysilane. Toss that name at a room of chemists and some will nod. The stuff has found its way into everything from car windshields to wind turbine blades. Out in the field, the need to stick things together and make materials last longer isn’t going away, so a molecule able to bond unlike substances becomes pretty valuable.
From working in materials science, I’ve seen the headache of getting a plastic surface to hold onto a metal finish or a coat of paint. Standard glues and primers only go so far—they don’t always offer the durability or moisture resistance modern products demand. Here’s where 3-Isocyanatopropyltriethoxysilane steps in. The molecule carries reactive isocyanate (–NCO) groups on one end and silane groups on the other. One side bonds with organic polymers like plastics or rubber, the other locks onto inorganic surfaces such as glass or metal. In simple terms, it bridges different worlds.
Let’s say you’ve got to bond a polyurethane adhesive to aluminum for an outdoor sign. Rain, temperature swings and time all conspire to peel that sucker off. Silane coupling agents like this one don’t just glue; they actually form covalent bonds at the interface, which secures a long-lasting hold. That extends the lifespan of the product and keeps failures to a minimum, which is good news for manufacturers and end users alike.
Glass fiber composites—seen in everything from boats to circuit boards—benefit from this agent. Without it, fibers and resins don’t cooperate, which leads to brittle, weak boards. 3-Isocyanatopropyltriethoxysilane acts almost like a handshake between fiber and resin, letting stress distribute more evenly through the matrix. Car makers use it for windshield adhesives, making sure adhesives actually grip the glass instead of delaminating after a few seasons. Electronics have caught on too; the semiconductor world depends on surface treatments that improve adhesion and seal out moisture so tiny circuits keep ticking.
Silane chemistry has its hazards. Isocyanate groups can trigger asthma and allergic reactions if inhaled or touched. From my time in the lab, gloves, proper ventilation, and face shields were standard whenever we worked with these compounds. I’ve seen reports of irritation even at low exposures. It helps to design safer workspaces—fume hoods, sealed transfer, clear emergency plans. Industry has begun to ask if less-toxic alternatives or more robust handling procedures should become the rule, not the exception.
Better education makes a real difference. People tasked with using these chemicals deserve solid, hands-on training—videos and written protocols only go so far. Audits and walk-throughs help identify weak links in handling and can cut the number of incidents. At the policy level, industry groups and regulatory agencies look at emerging health data to update recommendations, keeping everyone safer.
3-Isocyanatopropyltriethoxysilane keeps showing up in applications that demand performance under pressure. Yet, this shouldn’t become an excuse for ignoring the health side of things. Through better training, improved formulation—like adding stabilizers or using alternative chemistries—and smarter equipment, companies can keep innovation alive and staff protected. Every time a composite yacht shrugs off a storm, or a skyscraper window stays sealed after years in the sun, there’s a bit of clever chemistry at play—sometimes thanks to molecules like this one quietly doing the hard work.
Anyone who spends time around chemicals knows that safe storage plays a bigger role than just ticking a box for compliance. 3-Isocyanatopropyltriethoxysilane isn’t a household name, but in an industrial setting, it pops up often—adhesives, sealants, and surface treatment applications all use this stuff. So, it makes sense to spend a few minutes unpacking what proper care really looks like for this chemical.
This compound brings together silane and isocyanate in one molecule. Both pieces react with things you don’t want in the mix: moisture in the air or water from a spill turns this chemical into a sticky mess, releasing irritating fumes. Breathing those fumes or letting the product touch your skin almost always leads to health complaints, not to mention issues for people with respiratory conditions. Many companies highlight its acute toxicity. For anyone working with this silane, even a mild whiff ought to be a warning.
Experience shows that leaving 3-Isocyanatopropyltriethoxysilane in a poorly sealed drum for even a few hours causes crusting at the opening. That means tight, air-free storage matters. Store it inside a cool, dry, and well-ventilated building. Forget any hope of sticking it in a damp shed—this chemical reacts with humidity at a surprising rate, forming nasty byproducts.
Rooms that see temperature swings or direct sunlight aren’t candidates for safe storage. Sunlight helps speed up chemical breakdown, so covered storage protects the product and everyone around it. Solid containers—think lined steel or certain plastics—stay upright, closed, and clearly marked. Many facilities double-seal their storage to keep out intruding air. Labels should include hazard information and production dates, not just for good practice but also because shelf life shortens if not managed well.
Working in a paint plant, I’ve seen how poorly managed chemical storage can turn a bad day into a disaster. 3-Isocyanatopropyltriethoxysilane forms flammable vapors at room temperature. One forgotten spark—be it from static, faulty wiring, or open flame—can do real damage. No one should store this chemical near oxidizers or strong acids, which ramp up fire risks even more. It might sound simple, but a clear, designated area, marked “No Smoking, No Sparks,” often prevents problems down the line.
I once saw a small leak go undetected for a weekend—by Monday, the smell gave it away, and a sticky patch on the floor required expensive cleanup. Personal protective equipment isn’t optional. Splash goggles, chemical gloves, and proper respirators keep workers out of harm’s way during both storage and handling. If there’s a spill, good training means workers use absorbent materials and ventilate the space, instead of whipping out a mop and spreading fumes.
Tight in-house procedures help stop headaches before they start. Keeping logs, inspecting storage areas regularly, and rotating stock limits accidental deterioration and exposure. Accidents linked to this chemical almost never happen in shops that stick to these basic storage habits. Companies that invest in training also avoid costly incidents, regulatory fines, and—most importantly—injuries.
I’ve learned that safe storage isn’t about red tape—it’s about preventing harm and saving everyone time, trouble, and money. For 3-Isocyanatopropyltriethoxysilane, the best approach means sealed, labeled containers, a climate-controlled and dry facility, a plan for emergencies, and a staff that knows what danger smells and looks like.
3-Isocyanatopropyltriethoxysilane plays a big role in many manufacturing settings. Folks working in paint shops, labs, and factories probably know it as a key ingredient for improving how coatings stick to different materials. I’ve seen it used for prepping surfaces in construction and electronics. It acts like a chemical bridge that helps materials hold together. The science behind that glue effect sounds impressive until you read the warning label on a drum of this stuff.
Breathe in its fumes, and you’ll know right away something’s not right. I once helped a friend with a flooring job and the chemical smell made my nose burn and my lungs ache. Turns out this reaction is common. Studies show that inhaling 3-Isocyanatopropyltriethoxysilane can trigger severe respiratory issues—coughing, wheezing, and in some cases asthma. Folks with prior lung conditions need extra care here. If this compound gets on your skin, it can cause redness, itching, or painful rashes. I remember an industrial hygienist mentioning a coworker who developed a nasty allergic reaction that lingered for weeks after accidental exposure.
Eyes have it just as bad. Even small splashes may burn or injure the cornea. The risk jumps if people don’t use the right gloves, goggles, or ventilation. In one case report out of Europe, workers were hospitalized after a poorly ventilated workspace led to high vapor concentrations. Occupational safety bodies like OSHA rank this type of isocyanate as a hazardous occupational chemical that can sensitize people. That means the more someone’s exposed, the more likely severe allergies might develop down the road.
Medical literature and data from the World Health Organization back up these personal stories. Chronic exposure to isocyanates, including this one, links to long-term lung damage and skin allergies. Toxicologists explain that low doses over time can build up, making each encounter riskier than the last. Reports from Health and Safety Executive (UK) show that frequent contact in workplaces with poor controls led to higher asthma rates among workers.
Each country enforces safety rules about this chemical for a reason. OSHA provides set limits for chemical vapor levels. The European Chemicals Agency classifies it as hazardous, pointing out everything from acute toxicity to potential organ damage from repeated doses.
In real shop floors and construction sites, proper training changes the game. That means showing new workers the ropes when it comes to safe handling: using masks with filters certified for isocyanates, wearing chemical-resistant gloves, and setting up fume hoods or extraction fans. If work involves spraying or mixing, full-face respirators and protective suits make a big difference. I’ve seen places cut back on accidents just by locking up chemicals after hours and keeping emergency washing stations stocked and easy to reach.
Reporting near-misses matters too. Sharing safety stories between teams helps others learn what risks look like before something serious happens. Regular health monitoring—either lung function checks or skin screenings—keeps problems from going too far. Bringing in medical pros for onsite visits helps catch issues early, especially in shops that use these chemicals every day.
The reality is chemicals like 3-Isocyanatopropyltriethoxysilane hold up entire industries. Their benefits are real, but only when managed with respect for their risks. Personal experience tells me some shortcuts just aren’t worth the fallout, whether for your lungs or your long-term well-being. Staying informed and keeping every worker involved in safety shapes healthier outcomes across the board.
A few years ago, I spent long hours working in material laboratories. Chemicals come with baggage — they promise innovation, but handling them wrong brings real risk. 3-Isocyanatopropyltriethoxysilane gives people a jump in silicone bonding performance. Chemically speaking, it combines the high reactivity of isocyanate groups with triethoxysilyl units. From sticking composite layers to building sealants, this stuff gives engineers options. But that reactivity also catches human lungs and skin off guard, so limiting direct contact and controlling vapor exposure turned into daily tasks, not just lines in a safety manual.
Once, a colleague got a single drop onto a glove, didn’t change it, and regretted it with a red, stinging hand for two days. Nitrile gloves at least 0.4 mm thick made the difference — latex gave inconsistent protection, and vinyl couldn’t hold out at all. For each transfer or mix, I reached for double-layer nitrile, a face shield, and a chemical-resistant apron. Sometimes, people try shortcuts, but 3-Isocyanatopropyltriethoxysilane soaks in through skin and travels on the breeze, so skipping gloves or goggles courts medical trouble.
Cupboards and makeshift hoods don’t cut it. Isocyanates target lungs, not just skin. Properly rated fume hoods (with a minimum face velocity of 0.5 m/s) provide peace of mind. I saw the effects of poor air control: workers coughed, eyes stung, and everyone slowed down, fearing the next headache or asthma flare-up. Sensitization can become permanent, which means even low-level exposure later can trigger strong allergic or asthmatic reactions. Respirators matter here too; only organic vapor cartridges (marked with a brown label) block these vapors. Ordinary dust masks do nothing.
Knocking over a beaker in grad school taught me that speed and clarity matter more than who’s to blame. I hit the spill kit, scooped up the liquid with absorbent granules, then bagged the waste as hazardous. Delaying action with this chemical leads to bigger problems. Open surfaces pick up residue. Liquid seeps into table cracks, waiting for the unlucky person who uses the area next. Even a small splash on concrete or metal continued giving off fumes half an hour later.
On hot days, storage cabinets heated up, so I always checked that silica gel packets inside stayed dry. Moisture causes slow but relentless hydrolysis, building up carbon dioxide and corrosive byproducts inside the bottle. Rusted caps and distorted seals meant time to replace stocks. Using amber glass reduced the risk of sun-driven reactions. Keeping inventory strict, labeling boldly, and training new users paid off in our lab’s pretty uneventful safety log.
Complacency creeps in just when nobody’s watching. Including this compound in every chemical safety training session — using real stories, not just hazard pictograms — brought attention to its risks and practical steps. People remembered the hand rash or the breathlessness of a coworker. Supervisors running monthly check-ins, double-checking PPE stocks, and reviewing cleanup drills raised the bar even higher.
All the paper rules in the world don’t replace simple habits: changing gloves fast, replacing old fume filters, keeping areas clutter-free, and always closing bottles between pours. Each step cost a minute — but nobody in our group ever went to the hospital on my watch. That’s the safest endorsement I can give.
3-Isocyanatopropyltriethoxysilane never gets mentioned in everyday conversations—it's almost a tongue-twister. Yet, ask any chemist working with advanced materials or adhesives, and the term rolls off the tongue with confidence. This substance acts as a bridge builder in chemistry, helping products last longer, stay stuck together, or handle moisture better. Having spent time in manufacturing plants and R&D labs, I’ve seen how often this one compound pops up in requests and blueprints.
Car parts need to stick, seal, and stand up to bad weather. Rubber hoses, gaskets, and seals face heat cycles, oil splashes, and salt from winter roads. 3-Isocyanatopropyltriethoxysilane hooks rubber and plastic molecules to metal and glass—otherwise, these parts peel or crack. Nearly every rubber-to-metal bonded part rolling off an assembly line has probably felt the effects of this silane. Without it, carmakers would be changing their specs a lot more often.
Electronics live in small spaces and deal with plenty of heat and humidity. Tablets, phones, and computers rely on silane-modified adhesives or sealants to attach components and protect circuits. My time troubleshooting circuit board failures has shown the value of using silane coupling agents to stop delamination or corrosion over years of use. The silane supports a connection between glass fibers and plastic resin in circuit boards, locking components in place and lowering risk of service calls or returns.
Windows, façades, and concrete joints need serious weather resistance. Sealants and coatings with this silane help buildings survive the elements: water beads off, and surfaces stay tougher. I’ve toured job sites and seen how these sealants prevent cracks or mold, especially in climates that swing from humid summers to freezing winters. Without the right chemistry behind sealants, property owners face repairs and leaks far sooner than planned. Even insulating glass units—those double-pane windows—use this ingredient to keep argon gas inside and moisture out.
Composites—everything from wind turbine blades to lightweight car panels—lean on silane coupling agents. They help blend fiberglass or other reinforcements into resins, making parts strong yet lightweight. Factories using injection or compression molding stock up on silane for this reason. In the paint and coatings world, paint peel is a dreaded problem, especially where surfaces are slick or exposed. 3-Isocyanatopropyltriethoxysilane boosts adhesion, stretching the lifetime of industrial coatings applied to bridges, pipelines, and tanks.
Getting the most from this chemical means thinking beyond what the supplier lists on the label. Using the right application technique, mixing conditions, and amount can decide the success of a whole product launch. Over-application doesn’t just waste money—it sometimes weakens performance. Training workers on safe handling, since isocyanates require strict safety protocols, goes hand in hand with technical upgrades. Partnerships between chemists, production engineers, and safety officers always pay off. When everyone shares knowledge, you end up with products and infrastructure that quietly last longer—saving headaches, money, and materials down the road.
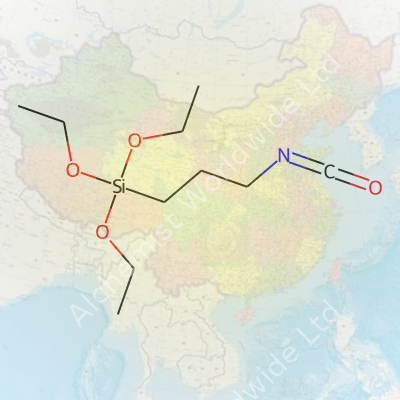
| Names | |
| Preferred IUPAC name | 3-isocyanatopropyl(triethoxy)silane |
| Other names |
3-Triethoxysilylpropyl isocyanate γ-Isocyanatopropyltriethoxysilane ICPTES Triethoxy(3-isocyanatopropyl)silane |
| Pronunciation | /ˈaɪ.soʊ.saɪˌæ.nə.toʊˈproʊ.pɪlˌtraɪ.iːˈθɒk.siˌsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 40372-72-3 |
| 3D model (JSmol) | `3d:JSmol__C[Si](OCC)(OCC)OCCN=C=O` |
| Beilstein Reference | 1465066 |
| ChEBI | CHEBI:87054 |
| ChEMBL | CHEMBL2183831 |
| ChemSpider | 5246363 |
| DrugBank | DB22000 |
| ECHA InfoCard | 03c762af-55de-4543-b863-601fdb84b2c1 |
| EC Number | 248-764-9 |
| Gmelin Reference | 1352216 |
| KEGG | C19316 |
| MeSH | D018015 |
| PubChem CID | 156415 |
| RTECS number | VV3045000 |
| UNII | 1UIA6M23JN |
| UN number | UN3334 |
| Properties | |
| Chemical formula | C10H21NO4Si |
| Molar mass | 247.36 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Amine-like |
| Density | Density: 1.025 g/mL at 25 °C |
| Solubility in water | Reacts with water |
| log P | 1.88 |
| Vapor pressure | 0.3 hPa (20 °C) |
| Acidity (pKa) | 13.5 |
| Basicity (pKb) | 5.64 |
| Refractive index (nD) | 1.420 |
| Viscosity | 2.5 mPa·s |
| Dipole moment | 4.34 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Danger |
| Hazard statements | H226, H315, H317, H319, H334, H335 |
| Precautionary statements | H315, H317, H319, H334, H335 |
| NFPA 704 (fire diamond) | 3-1-1-W |
| Flash point | 77 °C |
| Autoignition temperature | 275 °C |
| Explosive limits | Explosive limits: 1.3-9.5% |
| Lethal dose or concentration | LD50 (oral, rat): 2342 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 2340 mg/kg |
| NIOSH | WXV6435000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 0.045 ppm |
| Related compounds | |
| Related compounds |
Isocyanatopropyltrimethoxysilane 3-Aminopropyltriethoxysilane 3-Chloropropyltriethoxysilane 3-Glycidoxypropyltriethoxysilane |