
3‐Mercaptopropyltriethoxy Silane showed up in the chemical scene back in the mid-20th century. Chemical producers wanted to bridge the gap between inorganic surfaces and organic compounds. Early surface modifications hit serious roadblocks, with adhesion failing or environmental stability issues cropping up. Researchers realized that organosilanes could give them the chemical bridge no other compound managed. By the late 1960s, industries started testing and tweaking thiol-functional silanes, and 3-mercaptopropyltriethoxy silane became a real workhorse, especially after fiber-reinforced plastics and advanced composites called for stronger bonding agents. Big leaps in analytical chemistry and surface science since then have kept the interest alive, knocking down each wall one step at a time.
This compound goes by several names, from 3-Mercaptopropyltriethoxysilane to its common abbreviation MPTES or short-hand equivalents like MPTES or Silquest A-189. In the commercial world, it serves as a go-between for inorganic materials (glass, metals, ceramics) and organic resins, glues, and coatings. I’ve seen it show up in everything from adhesives for construction materials to catalyst supports in chemical R&D. You’ll spot it in supply catalogs, usually in clear or pale yellow liquid form, and its presence in advanced materials only grows as industries keep chasing stronger, more reliable combinations of diverse surfaces.
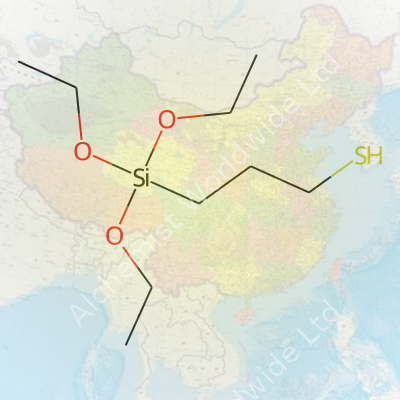
At room temperature, 3‐Mercaptopropyltriethoxy Silane shows up as a clear or nearly colorless liquid. It gives off a strong and distinctly thiol-like odor, not hard to miss in the lab. With a molecular weight just over 238 g/mol and a boiling point hovering near 238°C, it doesn’t evaporate too fast under standard handling. Its density stays around 1.05 g/cm³. The solubility in organic solvents is decent–think alcohols and hydrocarbons–but it hydrolyzes fast in water, forming silanols and ethanol, so storage matters. The thiol group likes to engage in reactions with metal ions, while the triethoxysilane ends hydrolyze and bind to siliceous surfaces.
Bottle labels usually print out key technical data: purity levels usually fall between 97 and 99 percent. CAS number 14814-09-6 pins down the product. Manufacturers highlight storage instructions, since moisture can trigger early hydrolysis, killing its effectiveness. You find hazard pictograms calling attention to its flammability and skin sensitivity risk. Containers recommend using the lot number for traceability in quality-critical settings. Batch specifications sometimes include GC-MS or NMR confirmation, since users in electronics and optical coatings care about impurities and side-products.
Making 3‐Mercaptopropyltriethoxy Silane starts out with mercaptopropyl chloride and triethoxysilane, running the reaction in the presence of an alkaline catalyst. Some manufacturers opt for mercaptopropyl alcohol, using classic hydrosilylation followed by chlorination to set up the silane coupling. Ethanol comes out as a byproduct. Quality control always tracks the moisture content, since water can hydrolyze silanes before they see use in composite manufacture. In real plants, I’ve watched process chemists lean on high vacuum for distillation, picking out pure fractions while catching stray acids or poorly reacted silanes for recycle.
What stands out about this silane is its dual-reactive nature. The thiol (-SH) head sticks to noble metals, metal oxides, and some transition metals, offering a direct binding route for catalysts or biosensing surfaces. The triethoxysilane group hydrolyzes in contact with moisture, forming reactive silanol groups that condense with hydroxylated glass, quartz, or silica. In my experience, surface functionalization with 3‐Mercaptopropyltriethoxy Silane transforms inert glass into a platform for enzyme coupling or advanced resin adhesion. Chemists often block or oxidize the thiol after anchoring the silane to the surface, adding another dimension for click chemistry, disulfide bridging, or linking to gold nanoparticles. Modification possibilities only keep expanding as more functional groups get paired or masked using standard protecting group chemistry.
Outside technical circles, you find this compound listed under several trade names: Dynasylan 3-Mercaptopropyltriethoxysilane, Silquest A-189, and various catalog designations from reagent suppliers. Synonyms like 3-(Triethoxysilyl)-1-propanethiol sometimes pop up, depending on the preferred chemical nomenclature system. I’ve tracked batches under these labels across years of work in analytical method development, finding it makes storage and purchase orders complicated unless you double-check CAS numbers and trade aliases.
Thiol silanes demand respect. Direct skin contact can leave rashes or irritation, partly from the reactivity of the thiol group, partly from traces of acidic byproducts. Labs working with this compound lean hard on fume hoods and splash-resistant goggles because repeated inhalation or accidental splashes hit fast. Material Safety Data Sheets flag it as a flammable liquid and require sealed, moisture-free containers. Emergency protocols advise against mixing with oxidizers. Training lab staff to spot early hydrolysis or off-smells in storage nips most problems in the bud. Strict inventory controls, regular air monitoring, and spill kits backed by fast cleanup procedures keep things safe even during scale-up operations in manufacturing.
Everywhere surfaces struggle to stick together or pass on chemical signals, this silane finds a spot. I’ve used it to activate glass slides for biosensors, coat silica for chromatography, and anchor gold nanoparticles for imaging. Industrially, its real strength lies in packing fibers for advanced composites used in wind turbine blades, sporting goods, and increasingly in automotive laminate parts. Its compatibility with epoxies and rubbers makes it a backbone of high-performance adhesives and sealants. Electroplating operations tune thin films using this compound. In microelectronics fabrication, wafer-level modification survives the rigors of etching or vapor phase processing thanks to the reliability of silane bonding. The boom in wearable biosensing has brought 3‐Mercaptopropyltriethoxy Silane back into biotech labs all over the world.
The research community doesn’t stop tinkering with this silane. My work with it stretches across surface energy testing and tailored linker chemistry for enzyme supports. Grants often target new hybrid materials combining carbon nanotubes or graphene oxide with silane-coated supports, hoping for next-generation batteries, sensors, or environmental catalysts. Analytical groups keep sharpening tools for surface characterization, using XPS, AFM, and contact angle goniometry, all pointing back to better application methods. Collaborations between industrial and university labs feed new patents in functional coatings, corrosion resistance, and environmental remediation. What used to look like niche academic interest now underpins several breakthroughs in sustainable chemistry.
Most toxicity findings show that acute effects focus around inhalation and dermal exposure rather than long-term systemic risks. Animal studies flag skin irritation and respiratory issues if concentrations climb high. The compound breaks down quickly in water, producing ethanol and silanols, so lab effluents rarely hold onto the parent molecule for long. In my experience, strict adherence to PPE, good ventilation, and proper waste management keeps exposures low. Some regulatory guidance points to potential aquatic hazards if large spills reach waterways. With ongoing research into occupational exposures, industrial operations monitor worker contact levels and lean toward substitution if less hazardous coupling agents work for a given process.
Next-generation electronics, medical diagnostics, and green chemistry keep this silane in play. I expect to see applications stretch into energy storage, flexible devices, and multi-phase catalytic systems. Demands for sustainable surface treatments and recyclable composites may push synthesis routes toward greener, less wasteful conditions. Pharmaceutical and biotech labs keep looking for safer, more efficient surface-coupling techniques, and as data pours in about long-term S-organic environmental impacts, cleaner modifications or better degradability could reshape how this compound gets used. The tools for measuring and tuning surface interactions improve year after year, promising tailored materials for just about every industry demanding tough, reliable interfaces.
Everyday products rely on smooth teamwork between different materials. In industries like automotive, electronics, coatings, and construction, 3-mercaptopropyltriethoxy silane stands out as an unassuming helper. People may overlook this clear liquid, but I’ve seen its influence on products as simple as painted panels or as critical as microchips. It helps things stick—literally.
I once toured a glass plant where technicians struggled with coatings that flaked from their windows. They switched to a surface treatment using a silane coupling agent and suddenly, the flaking stopped. 3-Mercaptopropyltriethoxy silane does what few molecules can: it holds organic and inorganic materials in a tight handshake that resists peeling, moisture, and even some chemicals. The molecule has a thiol (–SH) group on one end that attaches to metals and an alkoxysilane group on the other that bonds to glass or silica. In simpler words, it bridges the gap, plugging into both sides so nothing slides apart.
Reliable electronics start with strong connections between parts. In chip assembly, this molecule forms a thin, tough film between silicon and metal surfaces. I remember seeing engineers treat a wafer’s surface before building complex circuits on top. They counted on the silane layer to stop corrosion and reduce electrical resistance—small actions, but the yield of usable chips often hinges on these treatments.
Manufacturers load rubber with silica to extend tire life. The trouble comes with poor interaction between rubber and the filler; this leads to tires that break down faster and lose grip. By mixing in 3-mercaptopropyltriethoxy silane, rubber scientists found they could lock the silica to the rubber matrix and keep tread crumbling at bay. I learned about this from a friend in the tire industry—she credits the molecule for big improvements in wet-road safety and tread longevity.
Sealants used for windows, countertops, and baths look smooth on the outside, but gaps and poor adhesion can let water seep in or mold grow. 3-Mercaptopropyltriethoxy silane goes into these products to form flexible, watertight bonds between surfaces that usually repel glue. The resulting bond resists weathering, heat, and cleaning chemicals. I’ve seen contractors praise silane-modified sealants for keeping buildings dry through rough storms or high humidity.
I value products that deliver performance without putting workers or the environment at risk. This chemical can irritate skin and eyes, so companies invest in better ventilation, gloves, and training. Safer packaging and advances in automation limit direct handling, and new research seeks to lower emissions during use. These changes underline the industry’s responsibility to protect people while keeping products strong and reliable.
Materials science keeps raising the bar. 3-Mercaptopropyltriethoxy silane won’t solve every adhesion problem, but it shows how small molecules make big changes across countless applications—from safer cars to lighter electronics. Driving better design starts with choosing the right building blocks and respecting their risks. Supporting ongoing research, responsible sourcing, and data-sharing keeps industries resilient. Products work better, and people stay protected—goals worth investing in.
Anyone working with chemicals like 3‐Mercaptopropyltriethoxy Silane knows the drill: don’t leave a mess, keep things sealed, avoid heat and moisture. Those rules have roots in good reason, not just box-ticking. Use this stuff wrong, and you can end up dealing with strong odors, ruined products, or worse—exposure that could turn an otherwise routine day into a health scare. I’ve heard enough stories from people in labs and warehouses who let attention slip; suddenly, what was a useful additive turns into a problem that gums up entire workflows.
I spent several years moving between chemical warehouses and production floors. Over those years, I watched a new delivery of chemicals show up in the heat of summer. The supervisor trusted the storage room’s insulation, but didn’t check the thermometer during a string of heat waves. After a week, the vapors got so bad we had to dispose of several containers, racking up costs and headaches.
3‐Mercaptopropyltriethoxy Silane reacts with moisture in the air. If a drum seal leaks, it won’t just stink up the place—the chemical can start to hydrolyze. That means by the time you open the container, you might get clumps, strong sulfur smells, or lower quality. In my experience, keeping a real eye on seals, using clear labels showing open dates, and rotating stock stops product from breaking down. The best warehouses keep logs and require signoffs for every open drum. That traceability caught a few mistakes before anybody got hurt.
Forget fancy climate control talk. In practice, a dry, well-ventilated storage room beats a shiny new fridge set too humid every time. Store 3‐Mercaptopropyltriethoxy Silane in a spot away from direct sunlight. Warm, damp corners speed up its breakdown, so concrete floors that stay cool and racks that keep drums off the ground both help. Metal containers usually do the trick, as long as closures actually fit and resist corrosion from the chemical itself.
I worked one job where plastic caps swelled or cracked after a few months—nobody caught it until it leaked. Switching to reinforced steel closures stopped the problem flat. Those tiny changes prevent wasted inventory and cut risks to staff. That’s something you notice most if you’re unloading pallets, not sitting at a desk.
Regulations often get tossed around as red tape, but find yourself in a chemical spill without proper eyewash or a spill kit handy, and you realize why those laws exist. Following local chemical storage rules isn’t optional. Check your region’s hazardous material guidelines and use secondary containment like drip trays or bunds. A summary sheet of material safety data sheets by the storage room door helps everyone, from a new temp to the site manager.
Routine audits catch forgotten, half-used bottles or badly labeled jars. Once I helped with a cleanup where bottles sat so long that labels faded entirely—we found half solid, half sludge inside. Preventing that means enforcing inventory rotation and routine inspections, not just once a year, but as often as practical. People sometimes grumble, but peace of mind always wins when you know everything is in its place and every label matches the content inside.
3‐Mercaptopropyltriethoxy Silane isn’t dangerous if you give it the right respect. Good storage isn’t about overcomplicating process—it’s about paying attention. Anyone dealing with chemicals long term knows that those simple habits—sealing properly, labeling, keeping notes, cleaning up spills fast—make the difference between a smooth shift and a panic button call. Store it cool, dry, tightly closed, and you rarely face trouble. Skip any of these steps, and sooner or later, you end up paying the price—sometimes in product loss, sometimes in safety, occasionally both.
3-Mercaptopropyltriethoxy silane finds its way into specialty coatings and adhesives. Behind the impressive results of these products sits a chemical that calls for respect. The moment it comes out of the drum or bottle, anyone in the room will know from its sharp, sulfur smell. This alone hints at the need for careful treatment.
I learned to handle 3-mercaptopropyltriethoxy silane early in my chemistry career. Working with this compound taught me that gloves aren’t just a formality—the wrong choice can lead to skin irritation or worse. Nitrile gloves hold up better against this material than latex. Double-gloving adds a layer of confidence. Splash-resistant goggles never left my face, not only during weighing and mixing, but during cleaning as well.
The fumes can get overwhelming quickly, especially in a small lab or workshop. Fume hoods cut the risk in half. More than once, my colleagues and I noticed mild headaches after working in rooms with poor airflow. These symptoms disappear once you switch to handling this silane in a ventilated environment. Numbers back this up: inhalation exposure to organosilanes like this one increases risk of respiratory irritation and headaches.
The smallest unwashed hand can bring traces of silane into the body. When surfaces or hands carry even minute amounts, accidents wait around the corner. Eating or drinking anywhere near your workstation allows for contamination that usually goes unnoticed until symptoms show up. The safe bet is to keep all personal items out of the space and wash hands right after handling.
It takes only one splash to damage eyes. Liquid silanes, including this one, cause redness, pain, or even long-term harm. While goggles get uncomfortable in summer or humid rooms, they shield against accidental squirts or vapors. I watched a coworker skip goggles once; he needed medical attention in minutes. Afterward, no one in our group worked without proper eye coverage.
Improperly labeled bottles invite trouble. This specific silane forms reactive and smelly byproducts if leaks occur. Clear, permanent labels keep staff alert and informed even if there’s a shift change. Storing it in a cool, dry spot—away from acids, bases, or water—is a ritual in every safe lab. Leaky seals or loose caps lead to spills; corrosion-resistant containers prevent messes.
Spills escalate quickly without preparation. Absorbent mats and neutralizers sit nearby no matter how small the project. Individuals trained in clean-up methods limit the mess and protect themselves. Reporting accidents right away lets a team step in before vapors spread too far.
Knowledge saves lives in emergencies. Anyone who inhales, ingests, or gets silane on their skin should head outside, wash up, or flush their eyes. We kept emergency showers unblocked and knew exactly where they stood. Contact details for poison control and on-site first aid folks stayed on a visible poster.
Chemicals alone don’t cause harm; shortcuts do. Everyone in a shared workspace agrees on one set of rules for gloves, ventilation, and storage. The smallest change—a new piece of gear or accident—triggers new safety reviews. Experience, reliable data, and a steady respect for hazards push any project toward a safer result.
3-Mercaptopropyltriethoxy silane shows up a lot in polymer labs. People turn to it as a coupling agent, and it offers real benefits for certain materials. Its thiol group reacts strongly with metals and glass, while the triethoxysilane group sticks to stuff like silica and some plastics. But stories online sometimes make it sound like this chemical blends into every polymer out there. That’s just not the case.
I’ve seen folks get excited about 3-Mercaptopropyltriethoxy silane adding toughness to all sorts of rubber and plastic blends. The idea is simple: tie the organic and inorganic together so the final product holds strong. But if a polymer doesn’t have sites for the mercapto group to latch onto, the effects don’t always impress. Some hydrophobic plastics, like polyethylene or polypropylene, give poor reaction. These materials just can’t grab the molecule in a way that matters.
Epoxy resins, silicones, and certain rubbers do play nicely with this silane. Glass fiber composites often see real improvements. You get extra bonding at the interface, better water resistance, sometimes more strength. But work with other polymers, and you might just see a bunch of unreacted chemical going through the process, giving none of the benefits. Polyesters and polyamides often do better if they have enough compatible groups, but this is not one-size-fits-all.
I watched a team try this silane to fix delamination in a laminated flooring product using PVC and foamed core. After curing, they found surface stickiness and yellowing, not improved adhesion. This waste of time and materials stems from skipping careful lab checks. 3-Mercaptopropyltriethoxy silane reacts strongly in the presence of moisture and with surfaces that have available hydroxyl groups. If a polymer matrix is non-polar, nothing happens, no matter how much silane you throw at it.
Years of reports in industry journals back this up. Polymer chemistry handbooks note that using silanes with non-reactive polymers gives little effect. American Chemical Society and RSC resources both state the importance of surface chemistry. You see the difference clearly in sampled data: composites using the right silane show major improvement, while others barely register a change. This isn’t just academic. Improper use can increase cost, give environmental headaches, or leave a product below performance requirements.
The only smart way forward involves real testing. Check your polymer’s chemical structure. If available, look for surface modification methods before adding silane. Consider whether plasma treatments or primers could create sites for it to bond. Review studies on similar formulas, and track finished product results—not just lab tests, but the real use cases. Document what works, and communicate what doesn’t with your suppliers and tech teams. Getting good performance depends on matching up chemistry and processing conditions, instead of following chemical marketing buzz.
3‐Mercaptopropyltriethoxy silane shows up in all kinds of industrial blends — aerospace coatings, adhesives, plastic modification, and even some glass treatments. This chemical seems simple on paper, but getting the dosage right makes the difference between success and wasted resources. In my own experience working with silane couplers, overloading does nothing but hurt your margins, and going too light barely changes the final product.
The sweet spot for dosage usually runs from 0.5% to 3% by weight of the resin, filler, or substrate surface. I remember working with an adhesive manufacturer who shot for 2% by weight. The bonding strength rocketed after surface prep with 3‐mercaptopropyltriethoxy silane versus the untreated batch. An extra percent offered next to no gain, while cutting the amount left the bond weak. Too much of this silane can trigger agglomeration or just clog up downstream machinery.
It’s not all about numbers on a table. Surfaces loaded with silica, glass, or certain fillers respond better to higher silane levels. Someone adding a sulfur-cured rubber to a plastic blend will see benefits on the lower side of the range — around 0.5% to 1%. For glass fiber or mineral fillers, don’t skimp. Pushing closer to 2.5% can cut waste, raise bond strength, and help resist moisture—something every construction crew or composite panel builder wants when floors are flooded or heating cycles run for months.
Marketing pushes higher additive levels all the time, but real-world tests say otherwise. Studies from companies such as Dow have shown too much silane doesn’t mean stronger bonds. In some trials, excess silane forms a layer that actually blocks bonding, resulting in weaker joints or uneven surfaces. Nobody in coatings wants that headache — blistering and peeling are just around the corner. Environmental health teams also want you using as little as possible, since overapplication can bring safety or emissions worries.
Mixing techniques shape outcomes more than some realize. Steady stirring during silane addition keeps particle distribution even — clumping ruins interface quality. Drying time before proceeding to the next production step matters too. In one project, we shaved downtime by improving airflow but left enough moisture for silane to react with surface hydroxyls. If you jump ahead and bake or press too soon, you lose plenty of the molecular bridges that silane creates. Measuring pH and adding acid if needed pushes coupling reactions in the right direction.
Progress in materials comes from trial, error, and learning from setbacks. I’ve seen customers chase recommended dosage charts and end up lost with poor results. They adjusted procedures, worked with technical reps, and got back on track. The headline numbers — that 0.5% to 3% range — remain a good starting place. After that, make room for measuring, checking, and tweaking based on both lab and real-life production. Strong bonds, tighter composites, and lasting performance follow from attention to these small details.
Safety teams set their own rules on bringing new chemicals in. Every shift should enforce gloves, local exhaust, and eye protection during handling. Continuous training keeps crews aware of both efficiency and hazards. On the production line, simple checks — weighing ingredients, recording blending speeds, sampling finished lots — keep wrong dosages from slipping through. This isn’t just regulation compliance. It’s the way strong, reliable products reach the customer.
| Names | |
| Preferred IUPAC name | 3‐[Triethoxy(silyl)]propan-1-thiol |
| Other names |
3-Mercaptopropyltriethoxysilane 3-(Triethoxysilyl)propyl mercaptan MPTES 3-Triethoxysilylpropylmercaptan Triethoxy(3-mercaptopropyl)silane |
| Pronunciation | /ˈθriː-məˌkæp.təˌproʊpɪl-traɪˌɛθ.ɒk.si saɪˈleɪn/ |
| Identifiers | |
| CAS Number | 14814-09-6 |
| 3D model (JSmol) | `"CCO[Si](CCS)(OCC)OCC"` |
| Beilstein Reference | '1721396' |
| ChEBI | CHEBI:85334 |
| ChEMBL | CHEMBL1540070 |
| ChemSpider | 78492 |
| DrugBank | DB13840 |
| ECHA InfoCard | ECHA InfoCard: 100.033.535 |
| EC Number | 222-200-0 |
| Gmelin Reference | 87748 |
| KEGG | C19745 |
| MeSH | D02.886.521.395.400.760.500 |
| PubChem CID | 20838 |
| RTECS number | TP4550000 |
| UNII | N8N399R3V5 |
| UN number | UN3334 |
| CompTox Dashboard (EPA) | DTXSID5020683 |
| Properties | |
| Chemical formula | C9H22O3SSi |
| Molar mass | 238.41 g/mol |
| Appearance | Colorless to pale yellow transparent liquid |
| Odor | Mercaptan-like |
| Density | 1.056 g/mL at 25 °C |
| Solubility in water | Miscible |
| log P | 0.7 |
| Vapor pressure | <0.01 hPa (20°C) |
| Acidity (pKa) | 9.5 |
| Basicity (pKb) | 4.5 |
| Magnetic susceptibility (χ) | -77.0e-6 cm³/mol |
| Refractive index (nD) | 1.4400 |
| Viscosity | 1-2 mPa·s |
| Dipole moment | 2.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 589.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P301+P310, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P311, P312, P314, P321, P330, P333+P313, P337+P313, P362, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 74 °C |
| Autoignition temperature | 270 °C |
| Lethal dose or concentration | LD50 Oral Rat: > 2,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2600 mg/kg (oral, rat) |
| NIOSH | KXQ630000 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
3-Mercaptopropyltrimethoxysilane 3-Aminopropyltriethoxysilane 3-Glycidyloxypropyltriethoxysilane 3-Chloropropyltriethoxysilane Trimethoxy(3-mercaptopropyl)silane |