
Scientists have spent much of the last century searching for ways to bridge the gap between organic and inorganic worlds. From paints and adhesives to electronic components and coatings, almost every corner of industry has battled this challenge. In the 1960s, organosilanes started showing real promise, especially after discoveries pointed out just how effective their reactive groups could be in tailoring material surfaces. The introduction of 3-Mercaptopropyltriethoxysilane (MPTES) followed this era's drive for molecules that not only clung tightly to inorganic surfaces but also delivered reactive 'handles' for further chemistry. Watching MPTES transform, I see its lasting impact felt in labs and production lines over decades, as it carved out a place for itself by offering a thiol group that can be harnessed for everything from rubber vulcanization to gold nanoparticle conjugation.
MPTES shows up as a clear to slightly yellowish, pungent liquid, bearing the signature scent attributed to mercaptos. A single product can touch so many industries thanks to its dual personalities: one rooted in its triethoxysilyl anchor (finding affinity for silica, glass, metals) and the other in its thiol tip (latching onto sulfur-loving substrates or facilitating radical-based chemistries). I've worked with surface modifiers and adhesion promoters before, and the challenge always comes back to having a material that can bridge two worlds. MPTES keeps surfacing in practical guides and industry supply lists because it actually gets the job done, transforming surfaces reliably for polymer manufacturers, electronics assembly, or nanomaterial synthesis.
Every bottle of 3-Mercaptopropyltriethoxysilane brings together a mixture of practical characteristics. Molecular weight sits at around 238.4 g/mol; its density hovers near 1.056 g/cm³. It has a boiling point at about 238°C and tends to decompose before hitting a flash point, with a vapor pressure that’s manageable under normal conditions. The liquid structure guarantees quick reactions—too quick at times—if exposed to even a whisper of moisture in open air. Ethoxysilane moieties seek out water, starting hydrolysis and then condensation, so both storage and handling always deserve diligence. In the lab, I’ve kept MPTES stock solutions under nitrogen, sealed with parafilm and stowed in dry storage, not just because of manufacturer advice but after watching precious samples gel up upon careless exposure.
Suppliers usually market MPTES with purity above 97%. Labels typically read “3-Mercaptopropyltriethoxysilane,” along with batch-specific CAS number 14814-09-6, UN number 1993, and warnings about its volatility and toxicity. Certificate of Analysis documents will reference GC/MS confirmation, water content below 0.5%, and minimum active content guaranteed. In regulated spaces, the Safety Data Sheet comes standard, with detailed transport and environmental guidelines. For users like myself, the technical data makes it easier to compare across vendors and ensure a fair deal, even as product codes differ from one catalog to another.
At the bench or in the factory, making MPTES centers on reacting 3-chloropropyltriethoxysilane with sodium hydrosulfide, using non-polar solvents and close N2 or Ar protection. The process kicks off by stirring the halide and sulfide at controlled temperatures, letting the displacement reaction run its course, followed by careful washing and vacuum distillation. Others favor thiol-ene click approaches for specialized applications, feeding in propylthiol silane precursors with photo-induced reactions. Yields climb past 80% in scale-up runs with modern controls. Those aiming for ultra-pure batches might pass the crude distillate through molecular sieves and even column chromatography. Watching industrial chemists handle these operations taught me you can have great chemistry, but poor execution makes for lousy products.
Thiols have a reputation for being chemically mischievous, and MPTES fits right in. The mercapto group forms disulfides with oxidation or links covalently with noble metals like gold or silver. In polymer syntheses, this thiol acts as a chain transfer agent, lending control over molecular weights, or feeds into radical addition with acrylates or alkynes. Surface chemists graft MPTES to silica, glass, or even metals, forming robust siloxane bonds. With the triethoxysilane groups, hydrolysis and condensation create dense networks or single-molecule films. Changing the reaction conditions or swapping out solvents lets users tune both grafting density and orientation. In my experience with thin-film coatings, using MPTES gave reliable self-assembled monolayers that stick with minimal fuss, unlike so many other “magic” molecules that claim easy application but rarely deliver.
In procurement meetings and catalog browsing, MPTES goes by quite a few names: “SILANE, (3-MERCAPTOPROPYL) TRIETHOXY-”, “3-(Triethoxysilyl)propyl mercaptan”, and “γ-Mercaptopropyltriethoxysilane”. Some distributors simplify with “3-MPTES” or even “A-189” in reference to trade formulas. For those tracking HS codes or chemical registries, connecting all these synonyms keeps regulatory teams and buyers on the same page, especially in international shipments. I’ve spent too much time tracking synonyms between suppliers to underestimate the value of a well-labeled drum.
Working with MPTES never feels routine—even seasoned chemists treat it with respect. Skin and eye irritant properties pop up straight away, so gloves and goggles come out even for short handling times. Vapors can agitate airways and, in confined environments, flammable concentrations build up fast. GHS pictograms label it as both hazardous for skin contact and potentially harmful to aquatic systems. Most protocols require tight waste control, using certified containers and managing spills with absorbent materials rated for organosilanes, followed by specialized disposal procedures. During training sessions, I’ve watched newcomers underplay the risk, only to complain about sore throats and itchy hands by the end of the shift—lesson learned: MPTES handles safety on its own terms.
Surfactants, adhesives, semiconductors, and biosensors count MPTES among their essential building blocks. In rubber industries, MPTES speeds up vulcanization, producing more elastic materials that last longer under stress. Electronics depend on its thiol group to organize nanoparticles and add precise chemical handles to silicon chips or glass electrodes. The world of nanotechnology has little patience for unreliable molecular linkers, and every gold nanoparticle project I’ve seen relies on MPTES to create stable, functional surfaces. Manufacturers in paint and coating industries also exploit its ability to bind fillers to resins, boosting durability and chemical resistance. The breadth of its utility means that, regardless of the scale or end-use, someone is likely turning to MPTES as a go-to solution, not just an option.
Research teams haven’t slowed down exploring new chemistry with MPTES. The molecule is a favorite for academic labs developing self-assembled monolayers, advancing diagnostics platforms, or fabricating advanced composite materials. Multipurpose surface modification lays a foundation for biosensors, drug delivery carriers, and even more efficient energy devices. Each application exposes unexpected challenges: hydrolysis, batch variability, or unwanted cross-linking. Drawing from personal lab experience, only rigorous small-scale trials and relentless optimization keep research projects on track before shifting over to pilot production.
Studies tracking the toxicity of MPTES have grown over the years, as concerns shift from workplace exposure to environmental impact. Acute dermal and inhalation toxicity surface early in animal and cell culture testing, showing potential for both irritation and longer-term consequences. Aquatic toxicity gets due scrutiny, leading environmental offices to set discharge thresholds and promote closed-loop application setups. Toxicologists keep circling back to byproducts like ethanol (liberated during application) and potential conversion products that might accumulate in soil or water. In my discussions with industry safety officers, a shared conclusion emerges: responsible handling starts not with after-the-fact mitigation, but with upfront engineering and airtight protocols.
Looking forward, MPTES stands on the threshold of an era obsessed with surface-driven technologies—everything from smart sensors and diagnostic chips to new coatings and hybrid composites. As green chemistry becomes less a slogan and more a regulatory demand, researchers now experiment with recyclable or non-toxic derivatives, exploring how modifications might keep strengths without baggage. Automation and robotics broaden the reach, leveraging accurate dispensing and process monitoring to cut losses and boost yields. Drawing on years of seeing specialty chemicals wax and wane in popularity, I see MPTES continuing to anchor itself in both old and new applications, so long as it evolves with the regulations, the science, and—at the end of the day—the people who trust its chemistry on their shop floors and in their research.
3-Mercaptopropyltriethoxysilane finds itself at the intersection of chemistry and modern industry. As someone who’s worked with scientists in laboratories and chatted with engineers in plastics plants, it's easy to see that real-world progress often comes down to little details like this: a silane coupling agent that helps stubborn materials stick together and behave the way we want.
3-Mercaptopropyltriethoxysilane goes by a mouthful of a name, but its role is pretty straightforward. It serves as a bridge between materials that usually refuse to cooperate. Knowing this chemical’s role helps anyone understand why tires hold together longer or why electronic devices keep shrinking without falling apart.
Materials like glass, ceramic, or metals on one side, and rubber or plastics on the other, don’t naturally bond well. Their surfaces have very different personalities, in a sense. In the world of manufacturing, this spells trouble during assembly. This is where 3-Mercaptopropyltriethoxysilane steps up. The silane part connects to glass or metal surfaces, while the mercapto (sulfur-based) group attaches to organic compounds, like rubber.
Think about assembling tires. The inner lining must bond firmly to the rubber tread and fabric reinforcement. Factories treat some surfaces with this silane to forge those bonds. American Chemical Society reports improved product lifespan and reduced tire failure due to advancements in coupling technology like this. On a more day-to-day note, when a car tire grips the road through rain and heat, there’s a little science like this at work in the background.
Electronics demand miniaturization, and that means chips, sensors, and circuit boards all become more fragile—unless the underlying materials are rock solid. Manufacturers use 3-Mercaptopropyltriethoxysilane to improve adhesion between silicon wafers and organic coatings. Going back to my early experience with lab development boards, I saw firsthand how flaky surfaces caused connection failures. The chemical link helps shield connections from humidity and mechanical stress, helping those circuits last longer even in harsh conditions.
It’s no secret that corrosion eats away at infrastructure. Protective coatings on metal beams, bridges, or machinery often have to fight off water and salt. Studies from corrosion engineering journals show that using coating primers with this silane boosts resistance. In practical terms: a bridge lasts years longer, reducing traffic delays and repair bills for everyone.
Any chemical with so much power in industry raises questions about health and environment. Protective gear during use is standard, as is proper exhaust ventilation. Regulatory guidelines in the US and Europe hold companies to strict handling and waste rules. From conversations with safety inspectors, it's clear—protecting workers comes before everything else, and most reputable factories follow these standards closely.
As demand grows for stronger materials and devices, and with pressure on companies to make products last without wasting resources, chemicals like 3-Mercaptopropyltriethoxysilane will keep getting attention. The real challenge: extending the benefits to new materials, staying mindful of health risks, and making sure tomorrow’s solutions actually outlast today's problems.
Anyone who has spent time in a chemistry lab knows the importance of safety, but working with 3-Mercaptopropyltriethoxysilane brings its own risks. This stuff can help make surfaces bond together in industrial applications, but it doesn’t play nice with skin, eyes, or lungs. I’ve seen talented researchers cut short their day because they took shortcuts or ignored warnings. Taking the risks seriously sets a better example—and you keep yourself healthy in the long run.
This compound gives off fumes that irritate the nose and throat. It burns if it splashes on skin or gets in your eyes. Based on its chemical structure, it reacts with moisture and can release ethanol, leading to flammable vapors in closed spaces. The strong thiol (mercapto) smell is more than unpleasant—it means inhaling too much could damage airways. For labs in schools or smaller companies, ventilation can slip through the cracks, making matters worse. I’ve had headaches after working with silica-based modifiers, even after following basic precautions; it drives home how quickly these chemicals make their presence felt.
Too many people stop at gloves, but for this compound, gloves with strong chemical resistance (like nitrile, not cheap latex) actually matter. Peeling gloves off and finding red, patchy skin underneath proves that point. I always double-check for goggles or wraparound safety glasses, since even tiny leftovers on a gloved finger can end up in eyes. A lab coat with cuffs keeps sleeves from riding up, which keeps splashes off your arms. Even in warm weather, skipping a lab coat isn’t worth the risk.
Unlike more common reagents, a normal surgical mask won’t cut it. I’ve seen labs running fume hoods at full tilt any time someone opens a bottle. Respirators rated for organosilanes are a smart investment, especially for frequent work.
Keeping chemicals dry helps avoid dangerous side reactions, so all containers get tightly sealed the minute pouring’s done. Labeling with both the full name and hazards (not just initials) keeps new staff from guessing, which reduces mistakes. Proper storage feels like an extra chore, but once someone accidentally mixes water into an open bottle and gets hit by hot fumes or splattering, they see why the rules matter.
If a spill happens, step away and keep others out until the area is aired out. Once, a bottle tipped in a hurry, someone tried to mop it up with paper towels, and the fumes lingered for hours. Better to grab absorbent pads and work slowly, wearing extra layers of protection. After cleanup, ventilate the room thoroughly. Waste needs a sealed chemical waste container, not a trash can. Washing skin right away for at least 15 minutes keeps burns to a minimum.
Reviewing safety data sheets before starting new projects makes a world of difference. Regular training, clear signage, and a willingness to ask questions—these habits keep labs productive and prevent unnecessary injuries. Improved ventilation and a designated chemical handling area set a strong foundation for those working with 3-Mercaptopropyltriethoxysilane. Respect for the risks, real experience with the consequences, and active teamwork define who stays safe over a long career.
Working with chemicals over the years, I’ve found there is always one rule that rises above the rest: respect what you store. 3-Mercaptopropyltriethoxysilane isn’t a household name, yet its applications cross many fields, from surface coatings to adhesives and beyond. An organosilane like this one brings value to labs and production lines because of its chemical reactivity—but that same reactivity deserves respect in storage.
Let’s be real—no one wants a spill, a ruined batch, or a much more serious event. This chemical brings flammability, a sharp odor, and can release hazardous gases like ethanol and hydrogen sulfide if mismanaged. Anyone who has ever dealt with a botched container or a fume-filled room knows the stakes. The chemical may react with moisture in the air, which builds up pressure or creates unwanted byproducts. Water vapor sneaks in through a bad seal, and all of a sudden, your bottle isn’t just a bottle anymore—it becomes a hazard.
Storing 3-Mercaptopropyltriethoxysilane in a dry, cool area isn’t up for debate. Moisture protection stands at the forefront. A tightly closed container, made from glass or compatibility-checked plastic, shields the contents from humidity and air. Sealing the original bottle every time reduces exposure. Oxygen can play tricks by helping along unwanted reactions, while humid air invites hydrolysis—the kind that not only eats into your material but also releases flammable vapors.
The space you choose has to stay below 30°C. I’ve made the mistake of keeping sensitive silanes too close to heat sources, forgetting that temperature swings from sunlight and HVAC systems make a surprise attack. High heat invites chemical degradation and pressure buildup. Ventilated storage cabinets built for flammable or corrosive substances earn their keep in any shop that deals in chemicals like this. Flammable liquid storage rules fit well here, minimizing the impact if something goes wrong.
Use containers with desiccant packs or inert gas blankets, like nitrogen, when long-term stability matters. Weighing and transferring chemical should always happen in a fume hood. The sulfur in 3-Mercaptopropyltriethoxysilane makes leaks hard to ignore, thanks to the strong odor—your nose offers an early warning system, but that isn’t the fail-safe professionals should count on. Skin contact and inhalation deserve PPE every single time: nitrile gloves, goggles, lab coat, and a clear workspace free of combustibles.
Patchy labeling or a mystery container sets the stage for mistakes. Always keep clear manufacturer’s labels and safety data sheets handy. I’ve seen teams skip these basics, betting on memory or routine, and the results aren’t pretty. Good documentation saves not just your reputation, but also your health.
Handling 3-Mercaptopropyltriethoxysilane safely rewards patience and care. Industry guidance from groups like OSHA and the National Fire Protection Association set out clear protocols for chemicals in this class. Storage and handling aren’t a guessing game. By treating moisture, air, and heat as constant adversaries, you can keep your operation safe and your inventory intact. In the end, building good habits—honoring both technical guidelines and lessons picked up in the field—keeps one’s workplace running smoothly and everyone safer.
Spend enough time around silicon chemistry labs and this name pops up a lot: 3-Mercaptopropyltriethoxysilane. It isn’t some obscure laboratory quirk—it plays a role in products people touch every day. Let’s break it down. The chemical structure looks like this: HS-(CH2)3-Si(OC2H5)3. The first part, HS, means a thiol group sits at one end. The “OC2H5” pieces? Those are three ethoxy groups attached to a silicon atom. Three carbons bridge the sulfur and silicon. That path turns this molecule into a bridge between totally different worlds.
I remember watching a coating technician prep glass with this stuff. The silane ends—the ones with ethoxy groups—bond tightly with glass or metal. After setup, the molecule grabs onto rubber, plastic or organic coatings with its thiol (sulfur) tip. Without 3-mercaptopropyltriethoxysilane, adhesives might peel, tires wouldn’t grip as well, and some electronics would call it quits too early.
The chemistry here isn’t just book-smart—it helps things stick where a handshake doesn’t work. Tests show that surfaces treated with this silane can double or triple their strength compared to untreated ones. It’s a strong solution for the world of plastics and rubbers, and one that matches scientific knowledge with the rough reality of daily life.
No chemical solves all problems on its own. Like any silane, moisture gets it going, but too much water starts a reaction too early. The ethoxy groups crack open in the presence of water and acid, turning the molecule into sticky silanols, which then grab glass or metal. If storage areas get damp, a whole batch can gel up or solidify before anyone has a chance to use it. I’ve seen large drums lose half their value just because someone forgot to keep a good seal or check the room humidity.
There’s another challenge in the air: Safety and handling. Most people notice the strong sulfur smell long before they see the liquid. Small spills leave a stench that hangs around, and some find out too late about skin sensitivity or eye irritation. Reliable personal protective gear and ventilation do more than tick boxes—they keep teams healthy and, frankly, willing to keep working with these chemicals.
Mistakes in handling and application leave failures in everything from construction materials to car parts. Experience shows that staff education makes a difference. Sharing real-life experience about moisture control—not just reading data sheets—pays off with fewer ruined batches. Quality testing at each stage reduces losses and keeps finished products up to higher standards.
Some teams look for better packaging and storage solutions, like inert gas blanketing or new drying agents, to get ahead of mistakes. Labs keep working on surface prep steps that make the mercaptopropyl group work even harder. By talking to end users and learning how final coatings perform long-term, improvements keep rolling in. That’s science, not just chemistry: Continuous feedback, tighter safety, and enough know-how to spot trouble before it grows.
Anyone who’s mixed up silanes in a lab knows the process isn’t just about pouring chemicals together. 3-Mercaptopropyltriethoxysilane brings a unique personality, mostly because of its mercapto (–SH) group. That sulfur atom makes it a sought-after player in the world of surface modification. For folks working in adhesives, rubber, electronics, or fiber composites, this is a material that sometimes serves as a bridge connecting organic compounds to inorganic surfaces.
Piling different silanes into the same mix doesn’t always end in harmony. Compatibility turns on reactivity, structure, and the demands of the finished product. For example, 3-Mercaptopropyltriethoxysilane plays well with aminosilanes, vinylsilanes, and epoxysilanes in controlled setups. These pairings often show up in glass fiber sizing or coupling agents in rubber compounding, lending toughness to tires or insulation to wires. If mixed with too much water, the ethoxy groups punch out early by hydrolysis, then start forming siloxane networks unexpectedly.
It’s critical to remember that not every material responds the same way. Excess water, acidic or basic settings, and uncontrolled temperatures sometimes push 3-Mercaptopropyltriethoxysilane into forming thick gels or unwanted precipitates. From my time in small-batch production, I’ve seen entire vessel walls coated with sticky residues simply because someone skipped the order of addition or didn’t seal the container fast enough. These lessons stick with you.
Industry tests show that 3-Mercaptopropyltriethoxysilane links strongly with silica—and sometimes alumina—on glass fibers, bringing up the bond strength between glass and resin. Its use in rubber compounding, especially with silica fillers, benefits from these chemical handshakes, making tires more fuel-efficient by reducing rolling resistance while increasing wet traction. According to journal articles in surface chemistry, this silane’s compatibility with other coupling agents, such as aminosilanes, can change depending on curing temperatures and the pH of the blend.
Many manufacturers rely on suppliers who provide technical support and purity certification, mainly because even small impurities in silane blends can explode what’s supposed to be a simple mix into a separation disaster. As a chemist who’s suffered through more than one sticky mess, I can’t overstate the need to test small samples before running the full batch, especially if the blend claims haven’t been proven in your own setup. There’s a trend toward silane compatibilizers and pre-mixed kits that eliminate guesswork, but transparency in technical sheets remains spotty. Labs that have robust QC processes save themselves a world of trouble.
Challenges crop up with storage, shelf life, and mixing order. Sealed containers, dry atmospheres, and accurate dosing all play a big role. Companies could be more open about what they include in their “compatibility” recommendations, drawing on real-life case studies rather than just theoretical compatibility charts. Bringing in more practical education during employee training on this front helps cut down material loss and boosts safety.
Efforts in new product development can pull from lessons learned by process engineers and lab staff. More collaboration in forums and technical conferences helps demystify the actual behavior of 3-Mercaptopropyltriethoxysilane around other materials. Getting it right doesn’t always come out of a textbook, but from sharing field-proven solutions and real failures—because in the end, hands-on experience balances out every technical datasheet on the shelf.
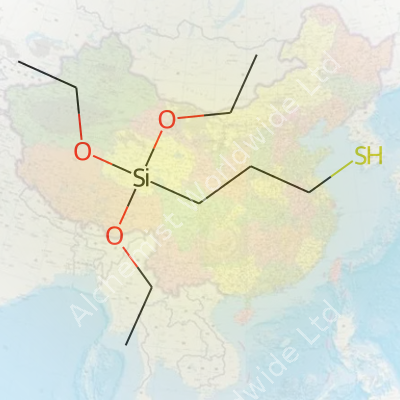
| Names | |
| Preferred IUPAC name | 3-(Triethoxysilyl)propanethiol |
| Other names |
3-(Triethoxysilyl)-1-propanethiol 3-Triethoxysilylpropylmercaptan MPTES SILQUEST A-189 γ-Mercaptopropyltriethoxysilane 3-Mercaptopropyltriethoxysilane |
| Pronunciation | /ˌθriː.mɜːˌkæp.təˈprəʊ.pɪl.traɪ.iːˈθɒk.si.saɪ.leɪn/ |
| Identifiers | |
| CAS Number | 14814-09-6 |
| Beilstein Reference | 803124 |
| ChEBI | CHEBI:85336 |
| ChEMBL | CHEMBL3211062 |
| ChemSpider | 89227 |
| DrugBank | DB14206 |
| ECHA InfoCard | 03bfa5fd-df01-4512-8a6c-1551d3685cae |
| EC Number | 216-382-4 |
| Gmelin Reference | 82238 |
| KEGG | C19325 |
| MeSH | D017262 |
| PubChem CID | 87105 |
| RTECS number | TP4550000 |
| UNII | D3O9U02UF0 |
| UN number | UN3334 |
| Properties | |
| Chemical formula | C9H22O3SSi |
| Molar mass | 238.42 g/mol |
| Appearance | Colorless to pale yellow transparent liquid |
| Odor | Mercaptan-like |
| Density | 1.056 g/mL at 25 °C (lit.) |
| Solubility in water | Slightly soluble |
| log P | 2.2 |
| Vapor pressure | 0.5 hPa (20 °C) |
| Acidity (pKa) | ~10.3 |
| Basicity (pKb) | 4.5 |
| Magnetic susceptibility (χ) | -75.0x10^-6 cm^3/mol |
| Refractive index (nD) | 1.440 |
| Viscosity | 2.5 mPa·s |
| Dipole moment | 2.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 492 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS08,GHS05 |
| Signal word | Danger |
| Hazard statements | H226, H315, H319, H335, H412 |
| Precautionary statements | P261, P280, P302+P352, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 2-2-1-W |
| Flash point | 73 °C |
| Autoignition temperature | 215 °C |
| Explosive limits | Explosive limits: 1.3–23% (in air) |
| Lethal dose or concentration | LD₅₀ (oral, rat): 2,298 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 2,960 mg/kg |
| NIOSH | GV2875000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
3-Mercaptopropyltrimethoxysilane Mercaptopropylmethylsiloxane 3-Aminopropyltriethoxysilane 3-Chloropropyltriethoxysilane 3-Glycidoxypropyltriethoxysilane |