
Back in the early days of advanced polymer chemistry, scientists searched for a silane coupling agent able to bridge the gap between organic polymers and inorganic substrates. Through persistent experimentation during the 1970s and 1980s, 3-Methacryloxypropyltriisopropoxysilane emerged as a standout compound. Its unique methacryloxy group, tethered to a propyl chain and terminated with three isopropoxy groups around a silicon atom, allowed researchers to surface-modify glass, metals, and ceramics with far greater stability compared to other silanes available at the time. Lab researchers recognized the potential for this molecule to link organic resins with mineral fillers, reinforcing composite materials. Over time, demand grew not just in academia, but also among manufacturers of coatings, adhesives, and even dental materials who valued the extra performance boost.
3-Methacryloxypropyltriisopropoxysilane, sometimes shortened in the industry to MPTIPS, often shows up as a clear, straw-colored liquid. It quickly earned a reputation as a molecular bridge. On one end, the methacryloyl group can polymerize with acrylic or methacrylic monomers. On the other, the isopropoxy silane moiety reacts with mineral surfaces. This dual action makes it a key ingredient for surface treatment or as a primer on glass fibers, minerals, and metal oxides. Manufacturers supply it in tightly sealed containers due to its sensitivity to moisture, with strict instructions for storage and handling. It often brings proven performance improvements to products in construction, automotive, electronics, and composites.
With a molecular formula of C13H28O5Si and a molecular weight hovering around 292.45 g/mol, 3-Methacryloxypropyltriisopropoxysilane boils at about 115 °C under reduced pressure. It carries a pungent odor — not unpleasant, but definitely noticeable if a container opens in a lab or plant. The density ranges from 0.97–1.02 g/cm³ at 25 ºC. Its refractive index falls around 1.423, making it easy to identify during quality checks. It dissolves quickly in common organic solvents like toluene, ethanol, and acetone, yet it hydrolyzes rapidly in water. Once hydrolyzed, the silanol group condenses with surface hydroxyls, a reaction crucial to its function as an adhesion promoter. Those working with the compound know not to let even a little water sneak into the container, or shelf life suffers.
Suppliers typically label this compound with identifiers such as CAS No. 80459-47-6, UN number for safe transport, and clear hazard labeling under international GHS rules. Labels feature the full chemical name, common synonyms, storage recommendations, and expiry dates. Material Safety Data Sheets (SDS) walk through the safest handling and emergency steps. Standard grades reach assay levels above 97% purity, with impurity specs for moisture and unreacted silanes. Barcodes and batch numbers make tracking straightforward for QA teams. Often, bottles carry tightly screw-capped lids with inner seals to block air and moisture ingress.
Manufacturing 3-Methacryloxypropyltriisopropoxysilane involves a reaction between gamma-methacryloxypropyltrialkoxysilane (like the methoxy or ethoxy version) and isopropanol under acidic catalysis. The process swaps the alkoxy groups for isopropoxy, usually by azeotropic distillation or direct alcoholysis. Skilled chemists control temperature and solvents to block side reactions, including self-condensation or premature hydrolysis. Reaction vessels need to stay dry and oxygen-free—one wrong move and the batch gels or clouds. Purification by vacuum distillation ensures a clear, high-purity liquid with robust storage life. Each lot gets checked for refractive index, GC purity, and silanol content to guarantee performance.
Exposure to moisture triggers hydrolysis of the isopropoxy groups, leaving behind silanol groups. These silanols condense with inorganic surfaces such as silica, alumina, or metals, fixing the organic methacrylate group at the surface. The methacrylate moiety later participates in free-radical polymerization. Anyone trying to boost the reactivity or compatibility might modify the backbone through substitution on the methacrylate end. Hybrid silanes sometimes include other reactive or functional organics to adjust properties for specialties like anti-corrosive coatings or dental adhesives. Scientists regularly tinker by co-formulating with other silanes or oligomers to widen the window of application.
Within industry literature and academic research, synonyms include 3-(Trimethoxy)propylmethacrylate silane, MPTIPS, and methacryloxypropyltriisopropoxysilane. Trademarked names might vary among big chemical suppliers, but the CAS number and core name appear consistently on documents and packaging. Sometimes users refer to it as a “methacryloyl silane,” which points to its dual-reactive structure.
Handling this silane calls for care. Skin or eye contact causes irritation, and inhaling vapors may upset the respiratory tract. Workers don gloves, goggles, and lab coats, following strict protocols on ventilation and spill containment. Even minor leaks invite moisture pickup, starting unwanted hydrolysis or forming gels. Plant managers keep stocks away from acids, bases, and open flames. Proper training in chemical transfer—using pumps, nitrogen blankets, and closed systems—lowers risk. Emergency eye washes and showers belong in areas where handling takes place. Waste handling stays tightly controlled: spent containers get rinsed and neutralized as per environmental regulations. Over time, improved PPE, automated systems, air monitoring, and worker training cut incidents way down.
No shortage of uses shows up for this compound. In composites, glass fibers gain new toughness after surface treatment, helping wind turbine blades, boat hulls, and sports equipment take heavier loads without delaminating. Adhesives and sealants show stronger bonds between organic polymers and inorganic fillers, be it in car engines or electronics. Electronic encapsulants see improved resistance to cracks and shrinkage. Dental researchers rely on it for resin-modified glass ionomer cements where biocompatibility and bond strength matter. Paint formulators apply the silane to boost wet adhesion, cut down on chalking, and lengthen product life. Artists rarely notice, but some specialized art restoration sealants owe their resilience to these molecular bridges.
University and corporate labs run constant experiments to adapt this silane for new surfaces and polymers. Composites research explores longer chain or branched variants to tweak flexibility or moisture resistance. Coating companies look for stable aqueous formulations (a tall order given the hydrolysis challenge). Biocompatibility checks happen, especially in dental and medical device testing, to ensure no harmful leachables reach patients. Teams also explore new curing mechanisms and visible light photoinitiators that pair with the methacryloyl group. As more industries demand tough, long-lasting hybrids, curiosity about this compound only grows. Large-scale studies explore the silane’s role in evolving areas like 3D print composites and flexible displays, blending chemistry with digital manufacturing.
Toxicologists have run acute and chronic animal studies to nail down risk factors. For the most part, this compound shows low oral and dermal toxicity, with no clear links to carcinogenicity at concentrations seen in commercial use. Eye and skin irritation can develop with direct exposure, so warnings on PPE stay justified. Long-term inhalation remains a gap, so industrial hygiene teams frequently monitor air levels. Wastewater treatment studies suggest the breakdown products do not accumulate, but caution about aquatic toxicity persists. Researchers watch out for any potential as a skin sensitizer, especially in closed indoor environments. Laboratory protocols evolve as new data rolls in, with updated SDS guidance widely shared across facilities.
Looking ahead, interest centers on smarter or greener variants of this silane. As composite material production keeps growing worldwide, the pressure is on to boost durability while cutting energy use and waste. R&D teams push for waterborne formulations, bio-based raw materials, and silanes that work at lower temperature or with new polymer chemistries. Renewable energy and electric vehicles lean into high-performance silanes to meet light-weighting demands. Medical device and dental teams continue looking for tweaks that bring greater safety without losing bond strength. Regulatory agencies press for more environmental data, prompting deep-dives into aquatics and biodegradation. The story of 3-Methacryloxypropyltriisopropoxysilane keeps unfolding as technology and global needs change. Each year brings new questions—and, often, very practical solutions no one could have pictured back in those early lab days.
Look at the construction of a bridge, the electronics packed inside your smartphone, or the replacement joints surgeons install. Each relies on materials that need to stick together and last under stress. 3-Methacryloxypropyltriisopropoxysilane often shows up in conversations on adhesives and coatings, because it helps different kinds of materials form a real bond. That bond doesn’t come easy, especially when dealing with combinations like glass and plastic, or metal and resin.
I’ve worked with coatings and adhesives in manufacturing, and one problem keeps popping up: two materials just can’t “talk” to each other. Glass rejects most plastic, metals shrug off many polymers. Silane coupling agents, especially this one, solve that problem. By acting as a sticky go-between with functional groups on each end, it lets the plastic grip the glass or metal and not let go. Once, while helping a packaging manufacturer deal with laminated films, adding this chemical improved the film’s peel strength and helped meet shelf-life demands set by retailers.
Manufacturers running composite production lines want fewer breakdowns and higher quality. They use 3-Methacryloxypropyltriisopropoxysilane because it does more than just stick. In fiberglass-reinforced plastics, for example, it treats the glass fibers. This small step before blending them in makes the whole batch tougher and less likely to crack under pressure. Wind turbine blades, car body panels, and sports equipment all benefit from that process.
Without consistent chemical bonding, you get micro-cracks, swelling, or early failure—big risks in industries with tight budgets and safety standards. According to reports from the American Composites Manufacturers Association, treating glass with this silane can raise the life span of components significantly, and lower the maintenance costs that nobody really wants to deal with.
The biggest surprise to me came in the dental world. Dental composites need to bond to tooth enamel and dentin, both tricky surfaces. Dentists use this silane in primers. This helps the filling not pop out after a few years. I’ve talked with dental researchers who track patient outcomes, and treated composites keep their bond longer, meaning fewer repeat visits for the patient.
Flip over to electronics, and you’ll spot a similar story. Printed circuit boards need reliable insulation and adhesion between tiny layers. Silicon chips need protective coatings that don’t flake when heated. Chemical stability and strong interface bonding make this ingredient popular among board manufacturers. Its methacrylate group hooks to organic polymers, while the silane group grabs onto glass or silicon surfaces, so the layers stick and data doesn’t get lost.
Every discussion about chemicals circles back to safety and sustainability now. As regulations such as REACH in Europe push toward greener chemistries, manufacturers work on lowering unnecessary emissions and waste during production. Some research looks into recycling silane-treated composites and lowering workplace exposure to volatile organic compounds. My own view: finding ways to keep these benefits while trimming the footprint remains a tough but important challenge.
People who never step foot inside a lab or factory rarely notice small molecules like 3-Methacryloxypropyltriisopropoxysilane. But it plays a powerful role in how durable, safe, and reliable everyday materials perform—quietly connecting worlds that otherwise refuse to stick together.
Chemists and materials engineers talk a lot about 3-Methacryloxypropyltriisopropoxysilane, or KBM-565 for short. It’s not just another mouthful of a compound. This silane pops up in specialty adhesives, coatings, and advanced composites. You walk past buildings and cross bridges, not knowing this molecule might silently keep concrete sealed or help glass fibers grab hold of resin. Plenty of high-performance surfaces quietly owe something to this silane coupling agent.
Chemical Formula: C16H34O5SiIUPAC Name: 3-(trimethoxysilyl)propyl 2-methylprop-2-enoate
To visualize this, start with the “methacryloxypropyl” end—a section with a double bond, built for reaction with acrylic groups. The other side holds the “triisopropoxysilane” group. Those three isopropoxy arms each connect to a central silicon atom. They aren’t there for looks. During material processing, these arms can hydrolyze, forming silanol groups that bond tough to materials like glass or metal oxides. That dual-purpose backbone lets the molecule stick two different worlds together: organic polymers and inorganic surfaces.
A simpler way to sketch the structure:
Plenty of chem students remember their first time handling a silane like this. I used KBM-565 in a lab on glass slide surface treatments. At first, I underestimated the prep—sloppy cleaning, just wipe and squirt the solution. But the slides with proper degreasing and that thin silane layer bonded like crazy to polymers, even after boiling water baths. That cemented my respect for these structures. It’s not just theory; these chemicals make interfaces last.
Performance gains appear in everything from better automotive headlamps to more durable wind turbine blades. Fields like electronics benefit, too, since proper adhesion keeps circuit boards intact under strain and moisture.
Experiments and published research show that a well-bonded interface gives products a longer life and keeps failures low. Composites using this silane formula stay robust under UV, salt, and heat. Testing often proves that without this coupling, micro-cracks open fast, water sneaks in, and delamination sets in.
In any industry, process matters. Consistent pre-treatment, the right humidity, and correct concentrations help silanes like this maximize their performance. Missteps—skipping a step, wrong temperature, too much water—lead to spotty bonding and lost value. My time consulting in production lines taught me to always double-check surfaces and protocols. Without the science and hand-on awareness, these powerful molecules don’t deliver what they could.
Chemists keep tweaking the isopropoxy groups and the chain lengths to better match adhesives or coatings for a wider range of materials. Green chemistry pushes for lower emissions and safer hydrolysis byproducts. From what I’ve seen, close collaboration between chemists, engineers, and technicians gives the best chance to harness the full potential of 3-Methacryloxypropyltriisopropoxysilane and build things that last longer for everyone.
Working in a lab has taught me that the main dangers often come from substances that seem routine on paper but pack a punch if ignored. 3-Methacryloxypropyltriisopropoxysilane fits that mold. This colorless liquid, common in adhesives, coatings, and glass treatments, comes with some real health risks. Its fumes can irritate eyes and skin, and even experienced chemists sometimes forget air quality gets bad fast if ventilation lacks punch.
I’ve seen firsthand what happens when chemicals get stored in the wrong place. This silane reacts to moisture in the air, breaking down and releasing substances that irritate the nose and lungs. Leaving a bottle uncapped in a humid storeroom eats away at its quality and turns the air sour. Sealing the container tight, keeping it dry, and storing it in a cool spot are steps that protect not just the material, but the entire crew’s health.
Anyone in charge of ordering or managing chemical shelves needs to pick wisely. Not all containers block out air equally. Plain plastic never does the job like glass or high-grade tin. Too often, I’ve watched people grab the nearest bottle without checking compatibility, only to find labels peeling or product leaking weeks later. A solid shelving system, with chemicals stored away from water sources, makes a huge difference. Avoiding direct sunlight and heat sources stops silane from breaking down faster than it should.
Comfort wins over safety in far too many labs. Thick gloves, goggles, and a solid lab coat look clunky but earn their keep the first time a splash happens. A labmate once ended up in the ER due to a splash of silane on bare skin, followed by quick swelling and redness. Quality nitrile gloves, tight-fitting goggles, and sleeves that fully cover arms and wrists stop chemicals from finding a way in. It’s not about following a checklist; it’s about going home without chemical burns.
A fume hood, even a small one, beats any open window in controlling vapors from silanes. I remember one workday where poor ventilation let a small spill hang in the air for hours, leaving half the room with scratchy throats and red eyes. A working spill kit—complete with absorbent material safe for silanes and neutralizing agents—keeps people calm and accidents minor. Never underestimate the value of regular drills. They get everyone moving fast in a real emergency.
Nothing beats clear, legible labels showing chemical names and expiry dates. Too often, faded print or lost stickers delay quick action. Fresh workers benefit from walking through a hands-on demo, learning not just the rules but the real-life reasons behind each step. I always tell new team members about actual incidents, not just theory. Stories stick, and people remember safer habits if they hear what can go wrong.
Better safety with 3-Methacryloxypropyltriisopropoxysilane doesn’t mean inventing new rules—it comes from respecting the basics each day. Strong containers, dry storage, good gear, fast spill response, and regular training save skin, lungs, and sometimes more. In my experience, a group that values these habits keeps labs quiet—the best sign nothing’s gone wrong.
The mouthful of a name—3-Methacryloxypropyltriisopropoxysilane—often shows up in labs full of resin and glass. Most folks outside the lab miss what it does. Every time I worked with composite panels, I leaned on silane compounds like this one to get materials that typically don’t stick together, to act like old friends. Imagine glass fibers and polymer resin—natural tension, right? One handles brittle impacts, the other flexes. Silanes build that bridge, giving you a material tough enough for wind turbine blades or strong flooring tiles.
Anyone repainting an old building knows the pain of peeling and faded colors. Silanes slip into the mix to bond paint to surfaces like glass, metal, or ceramics. They don’t just glue things; they defend against water seeping in, especially in humid spots or on industrial floors where durability matters as much as good looks. I spent plenty of sweaty hours in factory spaces where coatings would chip away unless these additives pitched in to make finishes tough enough for forklifts and storms.
In electronics, tiny scale means stubborn issues. Circuit boards crack when heat hits or moisture sneaks in. Here’s where 3-Methacryloxypropyltriisopropoxysilane acts as an adhesive promoter. Plastics hold tighter to glass or silicon, and devices keep performing day in, day out. Look around any modern device—phones, solar panels, EV battery packs—chances are, this compound has kept something bonded during wild swings from winter cold to summer heat.
Aerospace and auto engineers forever chase lighter, tougher, more fuel-efficient materials. Composite parts fill everything from bicycle frames to jet wings, but poor bonding kills their edge. By mixing this silane into resins, fiber surfaces stick better, hold more stress, and block out moisture. My neighbor, who builds kayaks for a living, counts on these improvements to keep boats from warping after a season in the water.
Handling silanes takes real care. They react fast with water, letting off alcohol byproducts that build up in closed spaces. After talking with industrial hygienists, I learned that poor ventilation during use can put workers at risk. Regular checks and good PPE solve most of that, but there’s also a push underway for alternatives with a smaller environmental footprint. The chemical industry, pressed by regulators and customers, keeps investing in low-emission choices and stricter safety measures.
There’s a quiet competition to replace old materials with composites that last longer, weigh less, and withstand harsher conditions. Silane coupling agents like this one anchor these advances. In my experience, early reluctance around price faded quickly when companies compared it to the cost of repairs and reputation hits from failed products. Industries don’t stay still—every year, new research gives us silanes tuned to faster production and greener use, without losing strength.
Progress depends not just on new molecules, but on how people handle them. Better training, improved ventilation, and careful process controls help lower risks. Real results come from both solid chemical science and the folks who put it to work, day after day. Using 3-Methacryloxypropyltriisopropoxysilane isn’t about following a recipe; it’s about building materials and devices everyone can rely on, in any weather, for years to come.
3-Methacryloxypropyltriisopropoxysilane, often called a silane coupling agent, pops up in labs and factories that care about strong adhesives, coatings, or composite materials. Just because it’s common in advanced manufacturing doesn’t mean it deserves a spot on your kitchen shelf. The basics: it gives off vapors that can irritate the eyes and respiratory system. Letting it touch your skin or eyes hurts and may cause lasting sensitivity. Breathing in the fumes with no mask on, or getting it on dry, cracked skin feels miserable. It’s not the sort of thing you want to handle in sandals or without gloves.
I once watched a machinist rush through clean-up without gloves, thinking it wouldn’t make a difference. The rash he picked up stuck for days. NIOSH and OSHA have flagged organosilanes like this for skin and eye irritation, and in some cases, allergic reactions. Working around any chemical that smells “sort of strong,” or leaves you coughing, isn’t a sign of toughness. It’s rolling the dice with your health.
Some stories online tell of careless storage, open windows instead of proper ventilation, and skipping eye protection. The result—burned skin, watering eyes, a hacking cough, or worse, a chemical splash in the face that sends you to urgent care. Silanes can enter the body slowly through the skin or quickly through the eyes and lungs if safety slides. That risk never gets reduced by bravado or hoping for the best.
Smart workers put prevention first. Nitrile gloves block accidental contact. Protective splash goggles keep the eyes safe from stray droplets. A long-sleeved lab coat covers exposed arms. If you can smell strong chemicals, the room needs better ventilation—local exhaust hoods do a far better job than opening a window.
Every bottle gets a clear label. Secondary containers keep spills from spreading if one tips over. Every time I worked with chemicals in a shared space, we made sure the Material Safety Data Sheet (MSDS) sat within arm’s reach. This isn’t pointless paperwork; these sheets give specific advice for quick treatment after accidental exposure and spell out fire risks. I’ve seen a new hire skip the MSDS and wind up pouring chemicals down the sink—don’t do it. Proper disposal matters.
Outside the lab, having a working eyewash station and a safety shower within a short walk changes a minor incident to an easy fix, not a hospital run. Teams that discuss chemical hazards before work hits full swing lose less time and avoid surprise injuries.
Chemicals like 3-Methacryloxypropyltriisopropoxysilane offer a lot to science and industry, but they demand respect. Personal protection takes a few minutes of planning and simple supplies. Fresh air, gloves, and eye protection turn “just another shift” into a much safer day. No job pays well enough to risk burns, rashes, or worse, so building habits around chemical safety leaves no regrets—just peace of mind and smoother work.
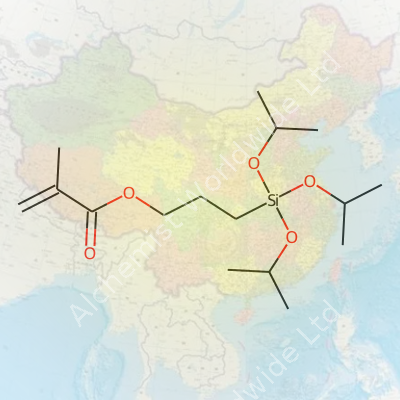
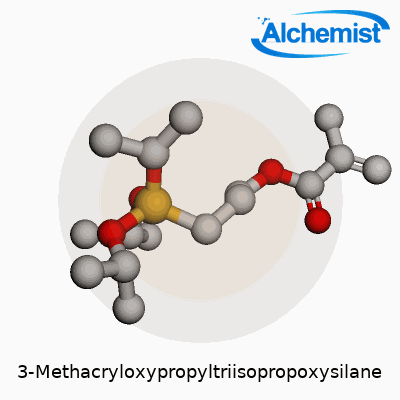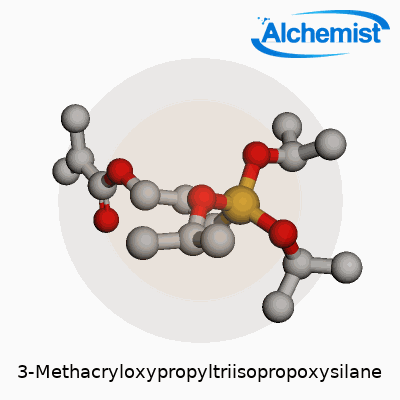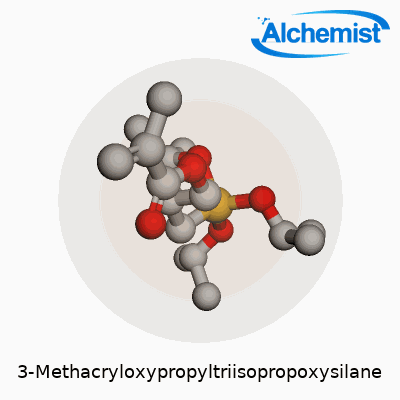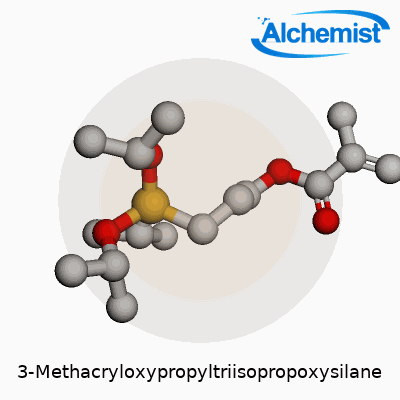
| Names | |
| Preferred IUPAC name | 3-(2-Methylprop-2-enoyloxy)propyltris(propan-2-yloxy)silane |
| Other names |
3-(Trimethoxypropylsilyl) methacrylate 3-Methacryloxypropyltrialkoxysilane 3-Methacryloxypropyltriisopropoxysilane Gamma-methacryloxypropyltriisopropoxysilane Methacryloxypropyltriisopropoxysilane |
| Pronunciation | /ˌθriː.mɛθ.əˌkraɪ.loʊk.siˈproʊ.pɪl.triˌaɪ.soʊˈprɒk.siˌsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 2530-85-0 |
| Beilstein Reference | 3531674 |
| ChEBI | CHEBI:89701 |
| ChEMBL | CHEMBL1545146 |
| ChemSpider | 20219633 |
| DrugBank | DB11240 |
| ECHA InfoCard | ECHA InfoCard: 100.151.154 |
| EC Number | 245-366-4 |
| Gmelin Reference | 1090664 |
| KEGG | C19582 |
| MeSH | D016690 |
| PubChem CID | 85818 |
| RTECS number | UF0950000 |
| UNII | 92U9F2724C |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID3058773 |
| Properties | |
| Chemical formula | C16H34O5Si |
| Molar mass | 324.52 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Characteristic |
| Density | 1.02 g/mL at 25 °C |
| Solubility in water | Reacts with water |
| log P | 2.99 |
| Vapor pressure | 1.6 hPa (20 °C) |
| Acidity (pKa) | 12.50 |
| Basicity (pKb) | pKb = 4.3 |
| Magnetic susceptibility (χ) | -8.0E-6 cm³/mol |
| Refractive index (nD) | 1.427 |
| Viscosity | 15 mPa·s |
| Dipole moment | 3.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 607.7 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | Precautionary statements for 3-Methacryloxypropyltriisopropoxysilane are: "P210, P261, P280, P305+P351+P338, P337+P313, P370+P378 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 108°C |
| Autoignition temperature | 220 °C (428 °F; 493 K) |
| Lethal dose or concentration | LD50 (Oral, Rat): > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose) Oral Rat: 6390 mg/kg |
| NIOSH | GVG06680 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Methacryloxypropyltriisopropoxysilane: "Not established |
| REL (Recommended) | 200 mg/m3 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
3-Glycidyloxypropyltrimethoxysilane 3-Mercaptopropyltrimethoxysilane Vinyltrimethoxysilane 3-Aminopropyltriethoxysilane |