
The journey of 3-Methacryloyloxypropyltriethoxysilane (commonly known as MEMO silane) kicks off in the chemistry labs of the early 1970s, right at the threshold of a new era for advanced polymers and composite materials. Researchers needed a bridge between organic polymers and inorganic surfaces, and the simple silicone-based coupling agents of the day fell short when faced with tougher applications. As surface science evolved, chemists focused on attaching functional groups to silanes, driving innovation in the ways plastics, glass, and ceramics could be permanently linked together. By following the trail of patents and academic studies, you see MEMO’s roots grounded in thermosetting resins, glass-fiber reinforcement, and advances in dental materials. The step of combining methacryloyl with silane groups opened up sturdy possibilities for long-lasting composites and water-resistant films, addressing durability challenges that had stymied earlier materials.
3-Methacryloyloxypropyltriethoxysilane stands out as a clear, nearly colorless liquid designed to couple organic and inorganic phases. Manufacturers highlight its suitability in glass fiber sizing, mineral surface treatments, and as a primer for adhesives and sealants. You usually find it in liquid form packed in airtight containers, since even minor moist air exposure can trip off hydrolysis reactions, reducing its effectiveness. The methacryloyl functionality supports polymerization with acrylics, and the triethoxysilane group offers reactivity with inorganic surfaces, including silica and metal oxides. The result—a product that delivers improved adhesion, abrasion resistance, and the sort of robust compatibility that matters to people making composites or paints that last.
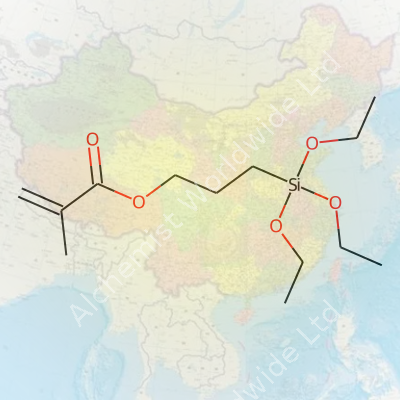
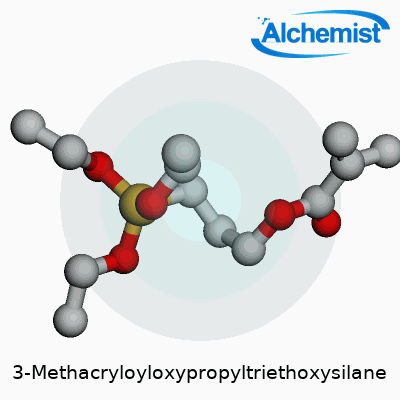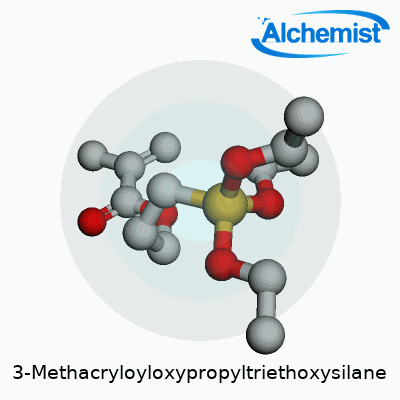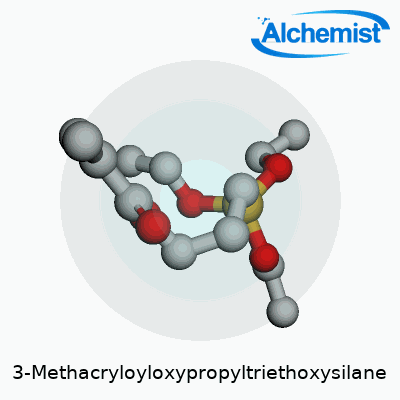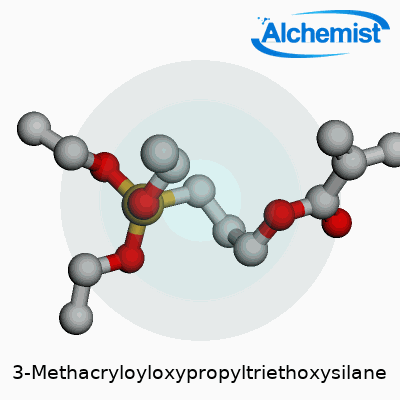
The material’s physical traits set it apart from generic silane coupling agents. Its molecular formula is C10H20O5Si, and you can usually spot its faint characteristic odor in the lab. The boiling point stretches north of 285°C, with a flash point around 110°C, which requires careful storage away from heat sources. The density lands close to 1.045 g/cm³ at 25°C. MEMO dissolves readily in organic solvents like ethanol or acetone, though it starts to hydrolyze if it sits in water. With its dual functional groups—methacrylate and silane—the compound straddles both organic and inorganic chemistry, giving it the bite to grip polymer chains and mineral surfaces alike. These features have pushed it onto the shortlists of engineers working with heat-cured resins or advanced polymer coatings.
When you pick up a drum of 3-Methacryloyloxypropyltriethoxysilane, labels list the minimum purity (usually not less than 98%), water content under 0.2%, and low levels of acid or methacrylic impurities. Density and refractive index are printed for quality assurance. Most suppliers provide batch numbers for traceability and fields for production and shelf-life dates. Shipping guidelines demand Hazard Class 3 compliance due to flammability concerns, and every shipment comes with a Material Safety Data Sheet covering exposure limits and emergency protocols. For customers using automated dosing systems, viscosity, freezing point, and solubility specs help nail down mixing times and handling temperatures.
Production of MEMO silane usually starts from methacrylic acid and 3-chloropropyltriethoxysilane. Under basic conditions, a nucleophilic substitution takes place, swapping in the methacryloyl group for the chloride. The process needs precise temperature control, tight exclusion of water, and careful post-synthesis purification; too much moisture invites unwanted hydrolysis of the ethoxysilane groups, leading to silanol formation and reduced shelf life. Large manufacturers rely on continuous distillation to pull out unreacted starting materials and low-boiling byproducts, aiming for the clarity and purity that keeps the product stable in storage. Handling raw materials like methacrylic acid comes with its own safety protocols, so you see heavy investment in operator training and equipment maintenance.
What makes MEMO unique in practice is its dual reactivity. The methacrylate end reacts with vinyl monomers in standard free-radical polymerization—think acrylic adhesives, dental fillings, and rapidly curing construction sealants. The triethoxysilane part undergoes hydrolysis and then condenses with hydroxyl groups on mineral, glass, or metal oxide surfaces, making a tough siloxane bond. This chemical handshake locks polymers to surfaces that normally resist any sort of sticky interaction. Researchers often blend MEMO with other silanes or crosslinkers to fine-tune the humidity resistance, flexibility, or adhesion profile for particular projects. Customization doesn’t stop at the lab bench—industrial finishing lines add small quantities of MEMO into resin baths or spray primers, improving bonding in reinforced composites and improving abrasion resistance under real-world, high-stress use.
3-Methacryloyloxypropyltriethoxysilane travels under several names. You might see it called MEMO, MPTES, or A-174 Silane in supplier catalogs. Some brands offer it as Silquest A-174, KBM-503, or even Z-6030, each tying back to the same core structure but often guaranteeing a particular purity or formulation tweak. Overseas markets use similar acronyms (KH-570 in China, Silo-METMAC in Japan). For buyers, double-checking the chemical structure always matters since a misspelled name or swapped prefix in a hurry could bring the wrong silane—leading to lost material and wasted effort during scale-up or R&D.
Lab veterans treat MEMO with respect due to its flammability and ability to irritate skin and eyes. Ventilation prevents buildup of vapors, especially during bulk blending and transfer. Chemical-resistant gloves, safety goggles, and splash shields belong on every work order. If a spill happens, workers use inert absorbents and ventilate the area thoroughly. Long-term storage requires tight seals, cool storage rooms, and labeling that doesn’t fade—nothing is worse than discovering a leaky drum of hydrolyzed silane on an inventory check. Emergency procedures include eyewash stations, drenching showers, and access to proper fire extinguishers (foam or CO2, not water due to risk of spreading the spill). Over the decades, the introduction of barcoded tracking, routine hazard drills, and third-party compliance audits have helped lower the rate of shipment and plant injuries tied to silane use.
The reach of 3-Methacryloyloxypropyltriethoxysilane stretches across construction panels, car bodies, wind turbine blades, and high-precision electronics. In the automotive industry, you find it enabling tough bonds between plastic lenses and glass covers, or supporting the durability of lightweight, composite-intensive body panels. In aerospace, manufacturers use MEMO to improve the fatigue resistance of carbon-fiber composites. Electronics engineers rely on its strong adhesion to glass or ceramic circuit boards. Even the dental sector values MEMO as a bridge between filler particles and polymer matrices in advanced cavity fillings and orthodontic adhesives. Paint formulators and flooring contractors have turned to MEMO to extend service life and waterproofing properties of coatings and sealants exposed to rough outdoor conditions. This seemingly niche chemical stands at the core of modern performance materials that ask for strong, long-lasting bonds in some truly tough environments.
Research teams keep chipping away at the limits of what MEMO can do. During long-term weathering studies, researchers track bond strength under UV radiation and daily temperature swings, aiming for formulations that keep their hold in desert, arctic, or coastal climates. Polymer scientists experiment with new allyl and epoxy-functional silanes, sometimes moderating the proportion of MEMO for sweet-spot performance. Much R&D goes into improving water and scratch resistance, as well as speeding up the cure rate without losing bond strength. In academic journals, you see data-driven studies measuring the optimal concentration of MEMO in fiberglass resin baths, or the exact pH required for a perfect hydrolysis/condensation balance. Companies tweak the product to suit new laws on VOC content and environmental safety, pushing the development of more benign catalyst systems and less hazardous solvent blends around MEMO. Some R&D groups blend computational modeling and real-world soak tests, compressing years of lab trial and error into more focused design work—all while tracking safety and toxicity with sharper tools than ever before.
Safety reviews give MEMO a mixed profile. At low concentrations, the chemical shows modest irritation to skin and eyes, as documented in both animal testing and real-life industrial exposures. Chronic inhalation studies on rats have guided workplace air limit recommendations, with fresh air ventilation and protective gear now standard in plants. MEMO’s hydrolysis byproducts—mostly silanols and methacrylic acid—raise questions about cumulative exposure for workers and downstream environmental impact, prompting stricter disposal guidelines. So far, long-term carcinogenicity remains unproven, but regulatory bodies classify MEMO among substances that need regular review and careful risk assessment. Industrial hygienists keep tabs on air and surface contamination, reinforcing a culture of “measure, clean, and educate” in production and handling environments to keep injuries and illness rare. Environmental researchers stay on top of wastewater standards, testing for methacrylate residues and siloxane fragments before any facility discharges run offsite.
Looking ahead, MEMO’s role may shift as industries chase better performance and lower environmental footprints. I see more demand for “green composites” in construction, where biopolymers need coupling agents to reach anywhere near the strength of petroleum-based resins. Wind energy, electric vehicles, and smart sensors all push for lighter, tougher, and longer-lasting materials. MEMO’s ability to crosslink surfaces and support complex material interfaces keeps it in the spotlight for new product rollouts. Updates to regulatory standards—driven by advances in toxicity testing and public pressure—keep pushing manufacturers to refine purity, handling, and disposal rules. Expect novel MEMO derivatives tuned for hot climates, salt spray, or reduced VOC emissions, as engineers search for ways to build more durable and sustainable infrastructure without sacrificing safety. The story of 3-Methacryloyloxypropyltriethoxysilane doesn’t stand still; it adapts along with technology and environmental needs, casting a wide influence on the materials that build the world around us.
Every time I hear about another mouthful of a name in the materials world, I look for the practical side. 3-Methacryloyloxypropyltriethoxysilane isn’t a trendy new ingredient. It’s a behind-the-scenes workhorse in industries built on glass, ceramics, plastics, and adhesives. Decades of scientific journals, industrial testing, and hands-on trial runs have shown that this compound changes how surfaces interact with each other. I’ve seen enough lab data and industrial field notes to trust its track record.
If you ask engineers about their everyday headaches, many will point to the trouble with joining things like glass and plastic. This silane stands out because it likes to play with both camps. With one end that’s happy with silicaceous surfaces (think glass) and another tailored for resins and plastics, it acts like a handshake between two stubborn parties. You start to realize its role goes well beyond the test bench – it shows up in dental repair work, windshields, and circuit boards. The difference is often in how long bonds last or how tough they become when weather hits hard.
Few people talk about the invisible failures in products. I’ve had my share of adhesives peeling when moisture seeps in. This silane tweaks the chemistry so resins stick to glass even under harsh conditions. Chemists use it to coat glass fibers before mixing them into composites, which means bridges handle stress better and sports equipment stands up to abuse. Printed circuit boards – the backbone of our electronics – carry this chemistry too. Better bonding means fewer headaches with delamination and less waste, since failures shrink significantly.
Here’s the rub: not every shop handles chemicals like this with the care it needs. The impact isn’t just on product strength but also on the planet and workers. Long exposure without the right gear can irritate skin or lungs. Training matters as much as the fancy glassware in labs. Regulation keeps an eye on this class of silanes, and industry updates reflect lessons learned from real incidents. It reminds me that smart chemistry respects both performance and safety.
I’ve noticed that global supply shifts, environmental rules, and green chemistry push manufacturers to rethink ingredients. Some companies now look for more sustainable production, better recycling after products reach the end of life, and fewer toxic byproducts. Open discussion between labs and production lines helps spot early warning signs for health and safety risks, while continued research slashes energy and raw material use. Schools produce chemists who value not just product strength but responsibility too.
People rarely call out what’s keeping their windshield bonded or dental fillings strong, but professionals in the field notice failures right away. Choices made before products hit the shelves affect reliability, repairs, and costs years down the line. I’ve read reports showing that switching to the wrong coupling agent costs money and reputation. Sticking with proven, well-understood solutions like 3-Methacryloyloxypropyltriethoxysilane, tempered with updated safety and sustainability practices, keeps industries moving and consumers happy.
3-Methacryloyloxypropyltriethoxysilane slips into conversations about high-performance materials with good reason. The silane part helps it bond with things like glass, metal, and stone. On the other side, that methacrylate group builds bridges with plastics, resins, and rubber. People in science circles call this “dual reactivity,” but to most of us, it means sticking stuff together that usually refuses to mix.
If you've ever worked in a lab or factory, you know nothing slows down a project quicker than a poor bond. This silane doesn’t just show up as a helper — it changes what’s possible. Its ethoxy groups react with moisture, hooking onto surfaces by forming sturdy silicon-oxygen bonds. There’s nothing delicate about the results: improved toughness, better weather resistance, and fewer failures in real-world tests.
Folks in construction and automotive industries lean on this molecule when they need plastics and metals to stay friendly with each other. It shows up in paints, adhesives, and even dental resins. My time consulting for a flooring manufacturer threw me into the weeds with silane chemistry. Floors see all sorts of abuse — water, mud, grease — and things like 3-Methacryloyloxypropyltriethoxysilane hold coatings fast, even after years of foot traffic.
Every good material can turn into a headache if mishandled. If moisture gets into the storage container, the ethoxy groups in this compound will react early, and you end up with a useless clump. Researchers looking for reliable performance know purity can’t be an afterthought. In my experience, companies willing to spend on dry, well-sealed packaging face fewer recalls and warranty complaints. Safety isn’t a buzzword around this stuff, either. Vapor can irritate eyes and lungs, so nobody skips the gloves and eye protection.
Studies back up what industry veterans have seen for years. Adding this silane to glass-fiber composites increases bending strength by up to 30%. In electronics, it cuts water absorption and boosts electrical insulation, reducing failures in devices where every short circuit can spoil a product launch.
Dental techs talk up its ability to lock ceramic crowns onto tooth resin. It’s easy to spot proof of its value, whether it’s fewer product returns or longer-lasting repairs. Even the tough weather in places I’ve lived, from the muggy Gulf Coast to icy Minnesota, didn’t faze treated materials during field trials.
No molecule solves every problem. The chemistry can’t do its job if workers skip surface preparation or if mixing ratios stray off target. Inconsistent application means lost adhesion. The price is another pinch point. For projects with tight budgets, the urge to swap in a cheaper alternative looms large. I’ve seen too many cost-cutting shortcuts lead right back to quality slips — and angry clients.
Getting the most out of 3-Methacryloyloxypropyltriethoxysilane comes down to careful use and sharp attention to the conditions. Companies get results when they invest in training, track humidity in their storage rooms, and run smaller-scale tests before changing recipes for big batches. Anyone dealing with legacy construction or repair work should weigh the cost of premature failures against the slightly higher upfront expense of high-purity silane. My advice: keep processes tight, don’t skimp on safety, and stay curious about quality — that’s where this compound lives up to its promise.
Folks working with 3-Methacryloyloxypropyltriethoxysilane, often called silane coupling agent, know this stuff isn’t your typical hardware-store caulk. It’s a chemical you find in plastics, resins, and sealants factories, and it earns plenty of respect in a lab or plant. Most people haven’t even heard of it, but the people who spend time around it learn quickly how unforgiving careless handling can be.
No matter how routine a task feels, direct exposure to 3-Methacryloyloxypropyltriethoxysilane carries real health risks—eye and skin irritation, lung issues if you inhale dust or vapor. Anyone on a production or research line ought to treat those warnings seriously. Chemical burns look dramatic but can start out unnoticed. Eye-wash stations and proper gloves don’t fix every problem, but any seasoned tech knows working unprotected can turn a routine shift into a hospital visit in seconds.
Extraction facilities and mixing rooms can get hot, stuffy, or downright sweaty in some corners. Moisture makes this silane break down and lose its punch, and high heat will spoil the whole drum faster than people realize. Supervisors and engineers worth their salt keep containers tightly sealed and store them in cool, dry rooms. We’re not talking freezer temperatures, but definitely out of the sun, away from steam lines, and never sitting open on a bench while lunch happens.
Nobody wants a mystery drum or leaking container on a busy shop floor. Most teams opt for HDPE bottles or lined steel drums with secure caps. This approach keeps the chemical’s integrity while sidestepping spills. Clear, honest labels matter just as much—hazard statements, proper chemical name, and the date it arrived. Back in my apprentice days, I saw coworkers grab the wrong solvent more than once. Good labels don’t just help in audits, they keep everybody safer, every day.
You can’t rely on nose or eyes to sense chemical vapor. Open windows or small table fans don’t cut it in a serious setting. Local exhaust systems that capture vapors right where the stuff gets measured or poured make all the difference. Personally, I never worked without a set of splash-proof goggles and chemical-resistant gloves. One whiff in the air lingers, and that’s your warning to reevaluate your setup.
Leftover silane and contaminated wipes often sit out, “just for a minute,” and then invite accidents. Trained crews call hazardous waste teams and store spent material in labeled containers, not open bins. Those crews keep spills from creeping out into soil or drains, protecting more than coworkers—they protect everyone who lives near the plant.
Chemicals like 3-Methacryloyloxypropyltriethoxysilane offer big rewards for industries, but they demand old-fashioned respect and discipline. Training never feels optional. Crew leads repeat drills, post reminders, and demonstrate safe habits. When leaders show attention to detail in storage and handling, it closes gaps where most accidents start. A team who cares about each other keeps a workplace safe, turning risky routines into smooth operations.
Most people never hear about 3-methacryloyloxypropyltriethoxysilane until a bottle of the stuff lands in front of them during a safety meeting. Lab techs or workers in the composites industry often handle it daily. The name alone draws suspicion, and plenty of new faces wonder if wearing gloves and goggles really makes a difference. Before tossing any product around a lab or shop, reading the safety data sheet (SDS) counts just as much as learning to use new tools.
The main worry isn’t the chemical itself in finished products, but how workers handle it raw. Breathing its vapor irritates the nose and throat. Liquid or vapor spills sting the eyes and burn the skin if left unwashed. Repeated contact sometimes inflames eczema or triggers allergic reactions, especially in folks with sensitive skin. Studies run on rats reported changes in organ weights at high doses. The jury’s still out about long-term cancer risk in humans; researchers reviewed available reports and didn't turn up clear links between this silane and cancer, but it pays not to gamble if you don’t need to.
The European Chemicals Agency classifies it as harmful if inhaled, and backs up the need for solid ventilation in mixing rooms or production spaces. Risk increases with poor airflow. Tossing toxic liquids into regular trash cans or pouring them down the drain only spreads the problem—trace amounts end up in water systems, affecting aquatic life downstream. That alone justifies extra attention to how we contain and dispose of unused chemicals.
Teaching new lab staff how to handle risky chemicals marks a big step in avoiding accidents. In real labs, gloves sometimes rip, bottles tip over, and distractions creep up during routine mixing. Even though the label might not scream “danger,” complacency causes more injuries than chemical properties themselves. My own early days running small batch testing brought close calls from splashes and forgotten masks—a harsh lesson to slow down and check gear before reaching for a beaker. Most injuries stay minor, but burns, rashes, or breathing problems can mean missed work and hospital visits if ignored.
Several studies from environmental agencies underline the wider effects. Trace silanes appear in effluent when companies flush untreated waste. Fish and invertebrates can’t metabolize some of these substances. Clean-up projects eat at company profits, spark regulatory fines, and hit communities living near industrial zones with unexpected pollution problems.
Safe handling isn’t rocket science, yet it doesn’t always become habit without reminders. Companies that set out clear protocols, run quick hands-on trainings, and keep hazard sheets visible see fewer incidents. Local exhaust systems, sealed mixing stations, and PPE help everyone leave work healthy. Experienced coworkers spotting bad habits early often prevent disasters. Disposal should always run through licensed waste treatment—never the regular dumpster or sink.
Some manufacturers experiment with alternative coupling agents that do less damage if spilled, though performance sometimes drops. Regulators and green chemistry advocates keep pushing for safer substitutes, but until those fully replace current formulas, handling practices remain the most reliable line of defense. No matter how common the activity, chemicals like 3-methacryloyloxypropyltriethoxysilane warrant respect—boring as it sounds, the safe practices you skip today shape tomorrow’s health, inside the lab and beyond.
Anyone who’s worked with concrete knows it’s tough to get materials to stick where you want. 3-Methacryloyloxypropyltriethoxysilane, usually called a silane coupling agent, pops up often in construction. It doesn’t just make two things “stick”—it helps bridge the gap between organic resins and inorganic surfaces. In glass fiber-reinforced concrete, a dash of this chemical helps bind fiberglass with concrete, cutting down water absorption and making things more durable. Plugging leaks or keeping a structure sound over years starts on the molecular level, and that’s the sort of job this compound does behind the scenes.
Anyone who repairs cars or follows automotive manufacturing trends runs into composite panels, lightweight plastics, adhesives, and sealants. There’s a demand for lighter materials to improve efficiency while keeping things tough under stress. This is where 3-Methacryloyloxypropyltriethoxysilane steps in as a coupling agent. It helps glass fibers mesh with polymer matrices in composite parts, like in bumpers or dashboards. That handshake between two different materials pays off in better crash resistance and longer lifespan. According to 2022 reports from markets such as MarketsandMarkets, automotive composites are expected to grow above 7% annually, and these silanes have an established place in those new materials.
If you’ve ever wondered what keeps the inner guts of electronics from breaking down due to heat, stress, or moisture, this chemical could be a quiet contributor. Printed circuit boards and encapsulants benefit from its ability to bond inorganic fillers with organic resins. This step boosts dielectric strength and reliability. With every push for smaller, faster devices, manufacturers fight problems with heat and moisture. Using coupling agents like this helps keep materials stable, so devices don’t fail sooner than expected. Considering that global electronics manufacturing is pushing $3 trillion, chemistry that extends device life isn’t insignificant for businesses or users.
Dental labs and offices are always on the lookout for stronger, longer-lasting fillings and crowns. In composite resins for dental repairs, 3-Methacryloyloxypropyltriethoxysilane helps fill the gap between inorganic fillers—like glass or ceramic dust—and the resin that binds them together. Dentists appreciate restorative work that stays put and resists stains or fractures. This compound’s role here contributes to patient satisfaction and reduces the need for repeat visits. The American Dental Association has published several position pieces on the role of effective adhesion in composite restorations, pointing to these coupling agents as essential ingredients in the mix.
Manufacturers put this compound into paints and coatings to get the right balance of toughness and weather resistance. If a painted surface seems to shrug off rain or heat, silane coupling agents might be part of the story. They improve dispersion of fillers and keep coatings attached through years of cycles between sun and rain. In adhesives and sealants, their utility keeps floors, tiles, and industrial components together in demanding environments. Watching a floor last for decades without peeling shows how these changes matter at home and in public spaces.
| Names | |
| Preferred IUPAC name | Trimethoxy[3-(2-methylprop-2-enoyloxy)propyl]silane |
| Other names |
3-(Trimethoxysilyl)propyl methacrylate gamma-Methacryloxypropyltrimethoxysilane A174 Silane, methacryloxypropyltrimethoxy- MEMO KH-570 |
| Pronunciation | /ˌθriː.mɛˌθæk.rɪˌlɔɪ.lɒk.siˌproʊ.pɪlˌtraɪ.iːˌθɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 2530-85-0 |
| Beilstein Reference | 1300634 |
| ChEBI | CHEBI:53253 |
| ChEMBL | CHEMBL185744 |
| ChemSpider | 157452 |
| DrugBank | DB11296 |
| ECHA InfoCard | 20f6e3a5-51c3-4424-9539-c92137b4c63e |
| EC Number | 210-419-6 |
| Gmelin Reference | 90847 |
| KEGG | C19296 |
| MeSH | D016461 |
| PubChem CID | 152815 |
| RTECS number | TZV54240M |
| UNII | R7IA8UMY3S |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID5040153 |
| Properties | |
| Chemical formula | C13H26O5Si |
| Molar mass | 248.36 g/mol |
| Appearance | Colorless to pale yellow transparent liquid |
| Odor | Characteristic |
| Density | 1.045 g/mL at 25 °C (lit.) |
| Solubility in water | Insoluble |
| log P | 1.99 |
| Vapor pressure | 0.08 mmHg (25 °C) |
| Basicity (pKb) | pKb = 7.6 |
| Refractive index (nD) | 1.429 |
| Viscosity | 2.5 mPa·s |
| Dipole moment | 3.24 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 689.7 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P333+P313, P337+P313, P362+P364, P403+P233, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 76 °C |
| Autoignition temperature | 230 °C (446 °F; 503 K) |
| Lethal dose or concentration | LD50 Oral Rat 8025 mg/kg |
| LD50 (median dose) | 7.8 g/kg (rat, oral) |
| NIOSH | RN 21142-29-0 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 0.1-2.0% |
| Related compounds | |
| Related compounds |
Methacryloyloxypropyltrimethoxysilane Vinyltriethoxysilane 3-Glycidyloxypropyltriethoxysilane 3-Aminopropyltriethoxysilane 3-Chloropropyltriethoxysilane |