
Chemists in the late twentieth century found new ways to link organic molecules to inorganic surfaces, chasing ever-tougher paints, sealants, and electronics. The push didn't just come from academic curiosity—manufacturing kept running into the problem of getting plastics, rubbers, or adhesives to stick to things like glass, metals, or ceramic. Somewhere in this uproar, 3-Methylacryloyloxypropylmethyldimethoxysilane entered the scene. Early patents from chemical giants show the scramble to turn ideas from labs into commercial batches. Over time, improvements in reaction pathways and demands for purer intermediates led to a purer, more reliable silane coupling agent. My experience reviewing patent filings and aging chemical catalogs tells me this molecule didn’t just appear overnight—it’s a product of years of trying different recipes until something stuck, both figuratively and literally.
3-Methylacryloyloxypropylmethyldimethoxysilane builds a bridge between organic polymers and inorganic surfaces, helping them adhere and share properties. On one end, the acrylate group wants to join with resins and plastics. On the other, the silane end bonds to glass, metals, or minerals. Manufacturers use it as a so-called “coupling agent” in adhesives, coatings, and sealants, aiming to solve classic headaches like delamination or poor water resistance. Those dealing with composites (one material layered on another) come across this name often as they look for better adhesion and longevity.
This molecule typically appears as a clear to slightly yellowish liquid, packing a strong, chemical odor that signals its reactivity. It is fairly volatile, evaporating if mishandled. Technically, its molecular formula is C11H22O5Si, and it weighs in at roughly 262.38 grams per mole. The duality of its structure governs much of its charm and challenge: the acrylate handles free-radical chemistry needed in polymerization, while the dimethoxysilane keeps things adhesive to tough surfaces. Its boiling point floats near 240°C, and it starts to hydrolyze in water, which means it’s touchy around moisture. People working around it or formulating products with it need to control humidity and storage conditions with care, or else the chemical may degrade and lose its punch.
3-Methylacryloyloxypropylmethyldimethoxysilane usually goes out with purity levels above 97%. Buyers often see technical data sheets listing its refractive index around 1.43 at 25°C, a density of about 1.03 g/cm³, and documentation about storage temperatures—typically in tightly sealed containers, cool, and dry. Labels warn of flammability, skin irritation, and possible sensitization, echoing years of accumulated safety data. MSDS sheets give crystal-clear instructions to avoid contact with eyes and skin and spell out what to do if a spill happens, hinting at why education on safe handling makes a difference for everyone working in industrial, academic, or commercial settings.
Labs typically synthesize this silane through a sequence that starts with 3-chloropropylmethyldimethoxysilane reacting with potassium or sodium salts of methacrylic acid or its derivatives. The process isn’t forgiving of impurities, so manufacturers keep a tight grip on reaction conditions—strict temperatures, dry glassware, and well-controlled pH. In larger settings, skilled technicians must run distillation to pull out side products, stabilize the batch against premature polymerization, and package it cleanly for shipping. Decades of industrial scale-up show that one false move, and unwanted hydrolysis leaves you with polymer lumps or a bottle of waste instead of a clean, reactive liquid.
3-Methylacryloyloxypropylmethyldimethoxysilane reacts vigorously with water, producing methanol and silanol groups that can then bind to inorganic surfaces—think glass, metal oxides, or even ceramics. Once it’s bonded, the acrylate group sits ready for free-radical addition in polymerization, which lets it anchor to resins, plastics, or rubbery compounds. Skilled synthetic chemists sometimes tweak this molecule, adjusting the methyl or methacrylate positions, to fit specialty needs. Its reactivity with amines or isocyanates enables its use in all sorts of crosslinking chemistries for adhesives, paints, and sealants. In my experience, you find this versatility cited in almost every major review of silane coupling agents over the last two decades.
Industry jargon breeds a collection of aliases. Chemists may know it as 3-(Methacryloyloxy)propylmethyldimethoxysilane, or abbreviate it as MAPMDMS. Product catalogs sometimes list supplier-specific codes like KBM-5103 or Z-6030. Regulatory documentation uses the IUPAC name or refers to CAS 65799-47-5. Recognizing these synonyms matters for safe handling, procurement, and regulatory filings. It’s easy to mix up similar silanes, so companies keep their material tracking razor-sharp to avoid costly errors.
People who handle this chemical must respect its health hazards. Vapors irritate skin, eyes, and lungs. Chronic exposure can lead to sensitization, making recurrent contact dangerous even at low levels. Factories and research labs set up fume hoods, gloves, and goggles as routine defenses. Emergency wash stations and detailed spill procedures crop up near every drum or bench bottle. Regulatory agencies, including OSHA and the European Chemicals Agency, flag its reactivity and potential environmental risks. Storage in original packaging, far from moisture and heat, reduces the chance of hydrolysis or fire. Mistakes in handling may result in severe chemical burns or long-term health consequences, giving every worker a stake in up-to-date training and vigilance.
Adhesion technology stands to gain the most from 3-Methylacryloyloxypropylmethyldimethoxysilane. Construction sealants, automotive paints, advanced electronics, and even dental resins depend on tough, persistent bonds between different materials. You see formulators reach for this coupling agent in polymer composites or specialty coatings to boost grip on glass fibers or tricky metal surfaces. Electronics manufacturers benefit by using it as a surface modifier to prep silicon chips for advanced packaging. Civil engineers add it to concrete additives, where it fights against moisture intrusion and extends the lifespan of structures. As composites step further into mainstream construction and transportation, demand keeps ticking up.
Research teams continue digging for better formulations, new crosslinking strategies, and alternatives with lower toxicity. Academic groups chase after greener, water-borne systems, trying to cut back on volatile organic compounds. Startups and major chemical manufacturers alike test slight swaps in the molecule’s backbone or side groups, chasing better weather resistance, flexibility, or biocompatibility. I’ve seen grant agencies funnel funds toward safer derivatives, particularly as markets expand in consumer electronics and biomedicine. Collaboration between universities and industry speeds up cycles from idea to application, letting end-users benefit faster from innovations.
Toxicologists remain vigilant about potential health effects. Studies show short-term exposure often causes skin and eye irritation. Long-term inhalation or repeated skin contact brings the risk of sensitization—once you get it, even small amounts can trigger severe reactions. Regulatory reviews cite animal studies where high-dose exposures raise alarms about respiratory irritation and organ impacts. Community health advocates keep pushing for safer labeling, tracking exposures in workers, and calling for more research into breakdown products. While the molecule doesn’t pose the acute hazards of more notorious chemicals, chronic effects and improper disposal still pose risks.
Demands from electronics, energy, and infrastructure promise a bigger market for smart coupling agents. Companies with a track record for clean manufacturing and lower-toxicity formulations will thrive as regulations tighten globally. Green chemistry movements keep pushing for less hazardous and more biodegradable alternatives, nudging big industry players to tweak their portfolios. Lab benches worldwide brim with new candidates inspired by 3-Methylacryloyloxypropylmethyldimethoxysilane’s winning formula—tough, flexible, and adaptable, but always facing the next challenge of safety, supply chain resilience, and environmental responsibility.
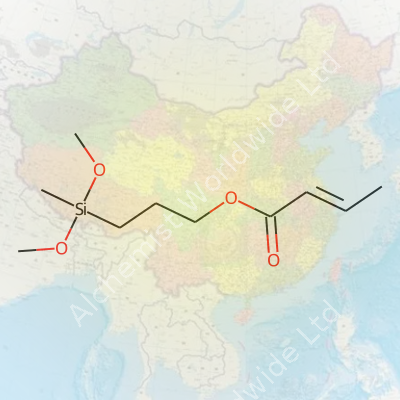
3-Methylacryloyloxypropylmethyldimethoxysilane sounds like a tongue-twister, but it makes a real difference in how many materials behave. This compound, found in many industrial and consumer products, carries a unique mix of acrylate and silane groups. That combination opens the door for it to link together very different types of substances—so plastics, glass, and metals suddenly play much nicer together.
Years of working with adhesives and coatings taught me that most problems pop up at the interface, where two different materials touch. Plastics don’t want to stick to glass, and metal shrugs off organic coatings unless you give them some help. 3-Methylacryloyloxypropylmethyldimethoxysilane acts a little like a matchmaker here. The acrylate end latches onto organic polymers, while the silane part forms strong chemical ties with surfaces like glass or metal. This dual-acting feature cuts down on peeling, flaking, and the early breakdown of composite materials.
Take windshields, circuit boards, or flooring. These everyday products use specialized coatings or adhesives, and failures can get expensive or even dangerous. This silane comes into play in sealants and paints for better water and chemical resistance—two enemies of lasting construction. It not only locks out moisture but also reduces yellowing and cracking in plastics exposed to sunlight. In the electronics world, this compound helps circuit boards handle harsh conditions and lots of thermal cycling without falling apart.
Factories push for tougher, longer-lasting products, but they don’t want extra steps that slow things down. Silane coupling agents like this one let manufacturers skip extra surface treatments or heavy primers. That saves energy and resources, two things always in short supply on a production line. Less waste and more efficient use of raw materials play into the bigger effort to reduce industrial impact on the planet.
Every chemical carries risks, and this one is no exception. People working with it need proper ventilation and should avoid skin contact. Silanes can react with water in the air, releasing small amounts of methanol that irritate eyes and lungs. Companies using these materials train their teams and install exhaust systems to cut risky exposures. Regulatory limits on workplace concentrations steer safer handling.
Chemists keep tinkering with these types of compounds, looking for ways to add features like antimicrobial effects or better flexibility in coatings. Better silane chemistries mean cheaper solar panels, stronger fiberglass, and longer-lasting plastics. The key is keeping up with testing to match health and environmental needs.
I learned early that trust depends on both science and transparency. Industry and research labs release safety data and keep improving ways to recycle and reuse composites made with these chemicals. The more open and careful the field becomes, the easier it is for people to feel safe with cutting-edge materials in cars, homes, and hospitals.
References:In labs where specialty silanes show up, 3-Methylacryloyloxypropylmethyldimethoxysilane often plays a role in binding organic and inorganic surfaces. Anyone working with organosilanes knows the importance of controlling their environment. I’ve seen firsthand what sloppy handling can lead to—skin exposure and ruined research, not to mention risks for people nearby. Health and productivity both take a hit if these chemicals leak or break down, which makes smart handling a must.
No one enjoys opening a drum only to be hit by a strong, irritating vapor. This silane can hydrolyze in humid air, and once moisture seeps in, reactivity can spike and cause unwanted by-products—clumping at best, fires or strong fumes at worst. Airtight containers matter; once opened, use the whole amount soon, or reseal and store it properly.
I always store organosilane products in cool, dry rooms. Excessive heat turns some of these compounds into ticking time bombs, speeding up breakdown and making them more hazardous. Keeping storage at around 2-8°C is a safe bet for stability, far from sunlight or heaters. Old containers should be double-checked for pressure build-up, and drum seals should stay dry and clean. It’s smart to keep a silica gel packet nearby, although checking the color change on the desiccant gives a clue if something went wrong with the seal.
I wear chemical-resistant gloves—nitrile, not latex, since some silanes chew right through softer gloves. A lab coat and full eye protection stay on until the work is done. Make sure the area has strong ventilation. Fume hoods are a basic defense, but I always crack a window or use spot exhaust if I’m outside standard benchwork. If a spill happens, absorbents like vermiculite work better than paper towels and cut down on vapor spread. Damp cloths cause more trouble by hydrolyzing the chemical faster.
Disposing of this silane goes far beyond a quick rinse. Flushing it into the drain is a dangerous shortcut—once these compounds meet wastewater, they react and release methanol vapor. Professional hazardous waste pickup is the answer; self-handling risks both legal fines and real environmental damage. Whenever containers go into waste bins, I punch holes in empty drums and clearly mark them so no one tries to reuse them by mistake.
Instant skin contact brings burning and discomfort. I’ve seen colleagues stall before washing off, and that’s a mistake. Immediate flushing of skin and eyes with water, for at least fifteen minutes, dodges lasting harm. Inhalation can cause coughing and headaches—if that happens, stepping out for fresh air and seeking a doctor’s advice works far better than “walking it off.”
People often view specialized silanes as less risky than acids or bases, but that’s wishful thinking. Regular safety training makes the difference. Standard operating procedures—written and posted near storage areas—keep everyone honest. In my experience, facilities that trim corners on protective gear or storage practices end up with higher incident rates and lower morale. Getting serious about chemical safety means treating every step with respect. This approach does more than prevent accidents; it saves projects, reputations, and people from avoidable harm.
If you have ever worked around industrial chemicals, names like 3-Methylacryloyloxypropylmethyldimethoxysilane jump off the label before you even glance at the safety data sheet. The word might wind a bit, but it's the effects on us and the environment that matter most.
I spent part of my early career in a lab, and the rule was simple: check the datasheet, double up on gloves, and keep your mask handy. This silane compound is a tool in many industries — adhesives and coatings, particularly — but that efficiency comes with a need for care. Its structure includes a methacrylate group, which sends up a red flag. Methacrylates have a reputation for causing skin and respiratory sensitization. In poorly ventilated spaces, the risk grows. The dimethoxysilane part can hydrolyze on contact with moisture, and the by-products, like methanol, are toxic and can seep through skin. A 2020 report from the European Chemicals Agency put this class of chemicals on their watchlist, urging tighter handling because of their irritation and sensitization potential.
In real terms, what have I seen? A colleague developed contact dermatitis after accidental splash and short-term exposure. Redness, itching, multiple doctor visits — it’s enough to turn anyone vigilant. Most cases do not result in dramatic headlines, but subtle, repeated irritation or asthma-like reactions can build up if gloves and goggles aren’t used or if a fume hood sits idle.
Straight to the point, this compound breaks down once it leaves the bottle and hits the open air or water. Silicon-based compounds often react with water, giving off silanols and, in this case, methanol. Methanol spills worry environmentalists for a reason — it’s toxic to aquatic life, and once it gets into a river, fish and invertebrates take the hit. While the silane portion tends to bind to soils and degrade, some metabolites don’t disappear as fast as we wish.
From my own rural experience — growing up near an industrial corridor — even small chemical runoffs led to odd smells by the creek, fewer frogs in spring. You might not pin it all on one compound, but the pattern is there. The EPA's risk assessments flag methacrylates for aquatic chronic toxicity, and the silane’s by-products linger.
Factories have more options than ever for protecting employees — real ventilation, not just a cracked window, makes a difference. Lab safety gear now fits better, and skin-friendly materials cut the risk of absorption. Everyone in the chain, right down to the janitorial staff, needs chemical training. The stuff I learned at a workbench rings true: treat unknowns with respect.
For the environment, catching spills before they hit drains matters most. Leak detectors and closed-system transfers, now standard in many places, keep a lot of nastiness out of rivers. Green chemistry is not just a buzzword; researchers have started swapping out these reactive silanes for less persistent options where they can. It costs more up front, but the savings in health and clean streams add up.
No chemical stays harmless just because it sits in a barrel. This one, like many others that keep our buildings strong and our products smooth, demands careful use, honest labeling, and eyes wide open for the subtle costs nobody sees right away.
Factories don’t run without a lot of surfaces coming together and staying together under pressure, strain, or corrosion. Silane compounds work a small miracle here. Mixing these chemicals into adhesives or sealants, manufacturers strengthen bonds between metals, glass, ceramics, and plastics. I’ve seen glass windows glued to aluminum car frames stay intact even after years of vibration and rain, thanks to a few drops of silane in the formula. This tool’s role keeps expanding, bridging gaps that older tech couldn’t handle.
Moisture ruins more than just a day; it wrecks infrastructure, paint jobs, and electronics. Silanes act as shields here. Concrete surfaces soak up silane treatments and shrug off water, stretching the usable life of bridges, buildings, and parking garages. The old rule—concrete crumbles in the rain—doesn’t bother people when silane gets used the right way. In my hometown, local government spent less repairing salt and frost damage after they treated exposed walkways with a silane-based sealer.
Paint peels, cracks, and yellows in the sun, but silane lets pigments and binders stick better. Paint companies add small amounts to get brighter, longer-lasting finishes, whether they’re covering cars, kitchen cabinets, or offshore oil rigs. Test results show silane-treated coatings hold their color longer against UV rays and rain. I tried an outdoor paint with silane last summer; my fence still looks as sharp as it did on day one.
Electronics don’t like the outside world. Moisture seeps into microchips and shorts them out, but a wafer-thin layer of silane blocks that attack. Semiconductor firms dip microchips in silane solutions to coat every crevice. Even phones with waterproof claims owe their strength to these coatings. It’s not some far-off manufacturing trick either—if you’ve carried a smartphone or used a solar panel lately, you’ve likely relied on silane behind the scenes.
Modern cars and airplanes fly farther on less fuel because their parts blend plastic and glass or carbon fibers. Silane bonds these materials together so layers don’t peel apart under heat, cold, or force. Line workers spray or mix silane right at the fiber-matrix interface. Without it, the future of lightweight vehicles would look much more limited. Energy saved from these high-tech composites lowers fuel bills and cuts emissions, one strong bond at a time.
Factories deal with dangerous dust and fumes all the time, but silane-based coupling agents let firms use safer water-borne paints and resins. Some older methods relied on solvents that could trigger health issues or fires. By switching to silane, workers breathe easier and risk drops. It’s a simple swap with serious payback—insurance costs shrink, sick days fall, and everyone feels safer on the job.
Silane technology isn’t stuck in the lab. Researchers test new tweaks every year to make these compounds friendlier to the planet, tougher under stress, and cheaper for small businesses. End-of-life questions pop up: what happens to old silane-treated buildings or products? Recyclers and chemists hunt for better answers, aiming to reclaim more material and put less burden on landfill sites. This pressure keeps innovation going and industry moving forward—often quietly, but always changing the game.
3-Methylacryloyloxypropylmethyldimethoxysilane isn't a substance folks want to leave sitting around. Coming across this silicone-related chemical in a lab or an industrial setting always sparks concern. Skin and eye irritation shows up pretty fast if it touches bare skin or splashes into eyes. Fumes can hit the lungs and throat on a bad day, so ignoring even a small spill can end poorly—for people, and for the place where it leaked.
Workers who’ve spent time around specialty chemicals learn early on that pouring leftovers down the drain is never an option. Local wastewater treatment plants can’t handle this stuff; their systems aren’t meant for unusual silicones, and letting them in can create problems in bigger water supplies. Tossing old containers in the normal trash breaks the chain of care too. Dumping them at a landfill turns small trouble into wider contamination down the road.
Instead, special disposal firms handle chemicals like this every day. These outfits know the paperwork, labeling, and transport laws. The Environmental Protection Agency tracks this process closely in most countries, making sure companies protect workers and neighbors by using the right procedures and containers. Most labs or factory floors keep spill kits and leak-proof storage on hand—once people get formal training, getting rid of dangerous leftovers grows into a careful habit.
Safety doesn’t happen by accident. Gloves, goggles, lab coats, and respirators—these build a layer between workers and a chemical that can bite back. Employees get instruction that goes beyond just “be careful.” They practice what to do if a bottle tips over, or if a hose bursts. Experience shows that even a little distraction, like hunger or frustration, turns fatigue into accidents. Frequent reminders and strong workplace culture go further than just rules.
It’s easy to overlook what happens after a chemical leaves the loading dock. Some companies cut corners trying to save a buck. Short-term savings don’t hold up when a spill poisons a stream or sickens a neighbor. Regular surprise inspections, government oversight, and industry transparency help keep temptation in check. People living near labs, factories, or shipping routes rely on everyone involved to take this seriously every time.
It feels good to see growing interest in green chemistry. Substituting dangerous chemicals for safer ones eases everyone’s burden. Switching to less toxic alternatives reduces the chance that a mistake creates a big problem for a whole community.
On the ground, proper storage, training, and clear labeling combine to minimize the risks. Firms send regular waste pick-ups to licensed treatment centers instead of waiting for stockpiles to build up. Management invests in best-practice systems, and they invite workers to report any weak spots. These steps don’t just tick boxes—they stop harm before it starts and pass on a cleaner, safer environment to the next set of hands.
It’s worth the effort. Disposing of 3-methylacryloyloxypropylmethyldimethoxysilane safely proves that a community values the health of its workers, neighbors, and land.
| Names | |
| Preferred IUPAC name | 3-(Dimethoxy(methyl)silyl)propyl 3-methylprop-2-enoate |
| Other names |
3-(Methacryloyloxy)propylmethyldimethoxysilane γ-Methacryloxypropylmethyldimethoxysilane 3-(Methacryloyloxy)propylmethyldimethoxysilane Methyldimethoxy(3-methacryloyloxypropyl)silane |
| Pronunciation | /θriː-ˈmɛθ.ɪl.əˌkraɪ.ləˌɒk.siˌprəʊ.pɪlˌmɛθ.ɪlˌdaɪˌmɛθ.ɒk.sɪˌsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 2530-85-0 |
| Beilstein Reference | 926139 |
| ChEBI | CHEBI:87177 |
| ChEMBL | CHEMBL185158 |
| ChemSpider | 10382873 |
| DrugBank | DB14624 |
| ECHA InfoCard | ECHA InfoCard: 100.190.372 |
| EC Number | 245-366-4 |
| Gmelin Reference | 95862 |
| KEGG | C19134 |
| MeSH | D02.455.426.392.368.330.500 |
| PubChem CID | 86774 |
| RTECS number | UB2975000 |
| UNII | XCU6K8K1G5 |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C11H22O4Si |
| Molar mass | 274.41 g/mol |
| Appearance | Colorless to light yellow transparent liquid |
| Odor | Characteristic |
| Density | 1.01 g/mL at 25 °C (lit.) |
| Solubility in water | insoluble |
| log P | 1.7 |
| Vapor pressure | 0.2 hPa (25 °C) |
| Basicity (pKb) | pKb: 6.5 |
| Magnetic susceptibility (χ) | -7.31×10^-6 cm³/mol |
| Refractive index (nD) | 1.430 |
| Viscosity | 5 mPa.s |
| Dipole moment | 3.0992 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 489.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -729.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3872 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H317, H319 |
| Precautionary statements | P261, P280, P305+P351+P338, P304+P340, P312 |
| NFPA 704 (fire diamond) | 1-2-1 |
| Flash point | 77 °C |
| Autoignition temperature | 225 °C (437 °F; 498 K) |
| Lethal dose or concentration | LD₅₀ (oral, rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: > 2,000 mg/kg |
| NIOSH | N.A. |
| PEL (Permissible) | PEL not established |
| REL (Recommended) | 3 ppm |
| IDLH (Immediate danger) | NIOSH has not established an IDLH value for 3-Methylacryloyloxypropylmethyldimethoxysilane. |
| Related compounds | |
| Related compounds |
3-Methacryloxypropyltrimethoxysilane 3-Methacryloxypropyltriethoxysilane γ-Methacryloxypropyltrimethoxysilane Vinyltrimethoxysilane 3-Acryloxypropyltrimethoxysilane |