
The road to developing 3-(N,N-Dimethylaminopropyl)aminopropyl methyl dimethoxysilane holds some of the milestones faced in the journey of modern organosilicon chemistry. Organic chemists in the mid-1900s, working on building silicon-based compounds for durable rubbers and adhesives, noticed that trialkoxy silanes offered a bridge between organic molecules and silicon frameworks. Decades ago, silanes were mostly about waterproofing or sticking stuff together. Technical innovation saw chemists adding functional side chains—like methyl groups or amine arms—to the silicon atom. With each tweak, new molecules promised stronger bonds or better reactivity. By the 1980s, the value of introducing tertiary amines as linking groups grew clear, especially for adhesives and coatings that must stand up to weather or chemical attack. Researchers pushed on, developing the specific compound we know today, combining both the methyl and dimethylaminopropyl features for multifunctional commercial use.
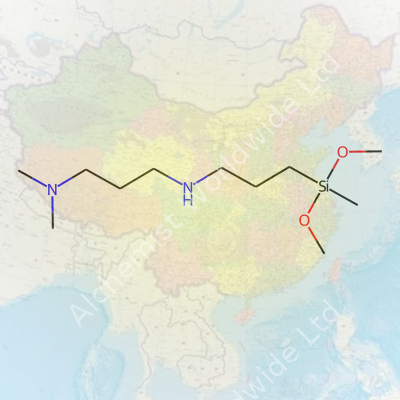
3-(N,N-Dimethylaminopropyl)aminopropyl methyl dimethoxysilane brings together two important functional worlds: the silane and the tertiary amine. The silane part links up with glass, metals, or other silicates, while the amine part can lock onto organic systems or enable cross-linking in epoxy or polyurethane networks. This compound shows up as a near-colorless to pale yellow liquid, recognizable by a distinct amine odor and a consistency that feels similar to common organic solvents. On the shelf, this molecule looks unassuming, but behind that appearance lies an ingredient making adhesives stick longer, giving coatings extra strength, and letting plastics blend with fillers or fibers that otherwise would not mix.
This silane’s molecular formula typically reads C10H26N2O2Si, with a molecular weight right around 246.4 g/mol. It holds up well in standard storage but begins to hydrolyze once exposed to moisture in the air, with the methoxy groups transforming into silanols and methanol. The boiling point hovers between 285°C and 295°C under normal pressure, while decomposition kicks in if heated past that. Its low viscosity and moderate volatility give it a manageable vapor pressure, which comes in handy during spray or dip applications. In water, it breaks down; in alcohols or glycols, it dissolves cleanly and reacts slowly, making it suitable for many industrial mixes. The tertiary amine part grabs onto acids and metal ions, which adds another layer of chemical versatility whenever modulating surface energy or tuning catalytic sites.
A technical data sheet for this silane will highlight its assay purity (usually over 97%), a moisture content kept below 0.5%, and a specific refractive index around 1.438 to 1.446. Labeling in most markets includes the United Nations identification number for flammable liquids and hazard pictograms that signal corrosive and irritant properties. There’s always a recommendation to store in tightly sealed containers, away from water and acidic or basic environments, since this keeps the product in active form and extends shelf life. Packaging ranges from drums to totes to smaller aluminum flasks, depending on user needs. Product safety data accounts for potential skin, eye, and respiratory hazards, supporting responsible handling by professionals during each step from warehousing to application
Most commercial syntheses of 3-(N,N-Dimethylaminopropyl)aminopropyl methyl dimethoxysilane start with methyl dimethoxysilane and a pre-activated 3-chloropropyl group. The process runs under an inert atmosphere—usually nitrogen—to prevent premature hydrolysis. An amination step brings in dimethylamine via an SN2 mechanism, with engineers tuning the temperature and pressure to maximize selectivity and reduce by-products. The reaction may involve a phase transfer catalyst, and purification often combines distillation with activated carbon to remove side products and color bodies. Production lines run continuous monitoring for water content to avoid gelling or loss of silane functionality, and the finished compound gets checked by NMR and GC to meet industry standards.
This silane reacts in several distinct pathways, thanks to its amine and silane duality. On one hand, the dimethoxysilane group hydrolyzes and condenses onto siliceous surfaces, forming durable Si-O-Si linkages that bind the molecule to glass, ceramics, or mineral fillers. On the organic end, the tertiary amine can participate in Schiff base formation or epoxide ring opening, especially valuable in epoxy adhesives or as a site for further functionalization. Chemists seeking to modulate hydrophobicity or charge density may modify the amine with sulfonates or quaternization, which flips the activity from nucleophilic to cationic. Under acid catalysis, the methoxy groups swap with longer-chain alkoxy or aryloxy groups, tweaking reactivity for specialty applications. The compound survives most neutral or basic conditions but breaks down quickly in mineral acids.
Chemical suppliers worldwide recognize this molecule under a host of synonyms. Labels may read N-[3-(Dimethylamino)propyl]-3-aminopropylmethyldimethoxysilane, or sometimes just DMAPA-mSilane for brevity. Leading companies list it under product codes like A-2110, Z-6610, or KBM-903. Research literature sometimes uses designations like DMAPAPMDMS. Looking out for these names prevents confusion when ordering or comparing technical specs across brands.
Lab safety teams pay close attention to the strong amine odor and corrosive nature of this silane. Direct skin contact causes burns, while inhaling vapor brings irritation or worse if safeguards slip. These risks push firms to prioritize splash-proof goggles, chemical-resistant gloves, and forced air ventilation any time silane hits the workbench. Facilities keep well-labeled spill kits with absorbent pads and neutralizers at hand, and large users sometimes run continuous air monitoring for ammonia-like vapors. Respiratory masks and fume hoods remain standard for weighing and mixing. Used containers and waste streams class as hazardous, so licensed disposal is a must. Fire safety protocols treat the compound as flammable, with careful grounding during transfer and storage.
Industries keep coming back to this silane for the way it bonds different materials. In glass-filled plastics, it serves as the go-between that lets polypropylene stick to glass fibers— boosting impact resistance and rigidity. Epoxy adhesives get a toughness upgrade by including this molecule in their primer blends, since the amine bonds well during curing, and the silane sticks to mineral or metal surfaces. Paint companies use it as a coupling agent, blocking the common problem of peeling from concrete or metal in humid conditions. Electronics manufacturers rely on it to modify circuit board surfaces before coating, raising adhesion or improving dielectric properties. Textile finishing and paper processing lines use the cationic amine portion to render materials more static-resistant or dye-receptive. Water treatment chemicals sometimes use the molecule to create charge-switchable surfaces, which catch and hold fines or organic grime. Its usefulness comes from doing more than one job in a single step.
Research teams worldwide keep pushing the boundaries for this silane, targeting both new applications and tweaks on existing processes. Material scientists investigate its ability to lock nano-fillers into polymer matrices, pursuing lighter, tougher composites for vehicle or sports equipment. Biotechnologists study if the amine functionality can anchor bioactive molecules, producing medical implant coatings that resist infection or promote cell growth. Researchers committed to greener chemistry explore routes using lower-toxicity solvents or catalysts, aiming to reduce the environmental load during silane production. Every year brings fresh patents describing new surface treatment blends or methods for more controlled hydrolysis. Some academic labs engineer analogs with longer or branched alkyl chains on the amine, checking if these bring better compatibility with advanced plastics or rubber recipes.
Long-term studies aim to pin down the threats this silane might pose on the factory floor, and, downstream, to the environment. Acute exposure causes strong skin and respiratory irritation, and animal studies draw clear links between high doses and mucosal damage. Chronic exposure data remains less plentiful, but regulatory agencies push for lower workplace exposure limits based on available results. Wastewater tests show that hydrolyzed silanes break down reasonably fast, yet some by-products, like methanol and formaldehyde, bring their own risks. Industry and public safety advocates campaign for routine monitoring and better closed-loop processing to limit occupational and environmental exposure. Material safety data sheets recommend emergency eye-wash facilities and detailed incident reporting, making this an area where responsible management separates the safe workplace from the hazardous one.
The future for 3-(N,N-Dimethylaminopropyl)aminopropyl methyl dimethoxysilane looks marked by continued diversification and tightening safety standards. As industries pull toward lighter materials, faster processes, and greener chemistry, demand for smart coupling agents will only grow. The switch from solvent-heavy coatings to water-based systems gives this silane new life, due to its reactivity and flexible compatibility. Digital manufacturing and printed electronics push researchers to craft ever more tailored surface treatments, many of which depend on high-performing aminosilanes. Regulatory agencies keep raising the bar for workplace safety and emission controls, which keeps driving investment into closed processing and safer alternatives. Market trends favor products offering multifunctionality, lower toxicity, and a reduced carbon footprint—all targets for future versions of this silane. Ongoing collaboration between academia, industry, and regulators will decide if these molecules stay at the margins or claim a central spot in tomorrow’s advanced materials.
Some chemicals live only in textbooks, but 3-(N,N-Dimethylaminopropyl)Aminopropyl Methyl Dimethoxysilane makes a real impact in manufacturing and technology. Let’s call it DMAPAPS for simplicity’s sake. This compound tends to appear wherever the world demands better bonding between organic and inorganic worlds—plastics and glass, paints and metals, resins and surfaces.
In adhesives and sealants, DMAPAPS works as a silane coupling agent. You wouldn’t think a tiny chemical could determine how well two different materials stick together, but it does. I’ve seen production floors where changing one ingredient in an adhesive made the difference between a strong bond and one that failed in days.
Electronics manufacturing depends on this compound for its ability to improve adhesion between silicon (the base of most circuitry) and organic coatings or encapsulants. Companies working on circuit boards mix in DMAPAPS when traditional adhesives just won’t stick well enough, especially as demand increases for miniaturization and reliability.
In paints and coatings, DMAPAPS strengthens the link between pigments, resins, and mineral fillers, which prevents flaking and peeling. Moisture resistance jumps up, too. My work with paint manufacturers taught me that a little boost in adhesion can shrink warranty claims and improve customer trust. People don’t want their walls or cars looking shoddy after just a season of weather.
The compound’s amine functional group gives it another trick: reacting with certain resins or reinforcing rubber. Rubber products made for cars, pipes, or industrial rollers pick up toughness and chemical resistance this way. Durable tires and reliable hoses often start with this silane backbone.
DMAPAPS plays a special role where materials face stress and harsh conditions. In construction, it improves how construction sealants, grouts, and coatings hold up under shifting weather, hot days, and heavy rains. Moisture creeping in can ruin a high-rise or cause costly repairs. Builders and contractors know that cutting corners on chemistry invites trouble later.
Fiber optics and advanced composites draw on this silane, too. I’ve talked with engineers who rely on it when building cables that have to hold up across continents and oceans. Loss of performance from water ingress or mechanical stress means downtime—something businesses can’t afford.
There’s always a downside. Silane compounds like DMAPAPS can irritate skin or eyes, and countries set tight rules for handling and exposure. Workers need proper training and personal protective equipment, including good ventilation and gloves. Producers who skimp on safety expose people to unnecessary risks, so oversight remains a must.
Environmental rules continue to tighten. Chemical releases, even trace amounts, spark investigations. Factories must invest in recycling, containment, or cleaner production methods. The best operations partner with local agencies and community leaders to share results and address concerns before accidents happen.
DMAPAPS stands out because it helps some of the toughest products meet high expectations without calling attention to itself. Most folks never hear about these chemicals unless something goes wrong. By focusing on safety, responsible sourcing, and clear lab practices, companies can keep unlocking the benefits while staying above board. That way, manufacturers keep customers happy, workers stay safe, and the industry builds a better reputation.
Encountering harsh chemicals in day-to-day work reminds me how fast a regular task shifts into a health emergency. The label on a container speaks volumes—corrosive, flammable, toxic—yet it pays to look for the full Safety Data Sheet (SDS) every time. Over the years, I’ve seen that trusting memory over documentation often leads to trouble. Shortcuts and “it’s probably fine” only stretch luck until it snaps.
Gloves and goggles feel uncomfortable in a hot lab or workshop, but skipping them sends too many people to the emergency room. I learned quickly that splash burns from acids or alkalis turn minor distractions into lingering pain. Using the recommended gloves—nitrile, neoprene, not just anything lying around—blocks more than just direct contact. Pulling on a face shield during transfers even when you think nothing spills builds habits that shield the skin and eyes from sudden accidents. Well-fitted lab coats and chemical aprons might not earn style points, but they save your regular clothes and cut down on direct exposure risks.
Every job site has its own quirks, but poor ventilation creates a trap for fumes. Whether working in a garage or a lab, opening windows and using fume hoods clears airborne dangers that nose and lungs don’t always detect right away. I’ve been in basements where a whiff of solvent lingers for hours because one fan just circulated instead of clearing the air. It helps to keep chemicals tightly capped, and away from heat or sunlight, to cut down on leaks, evaporation, and dangerous reactions. Separating incompatible substances—acids from bases, oxidizers from fuels—avoids fiery messes; shelves carry labels and containers stay upright for a reason.
No one expects a bottle to tip, but once it happens, preparation matters more than speed. Keeping spill kits stocked and close makes the difference between an annoyance and a disaster. I practice finding the eyewash and safety shower stations with my eyes closed. Seconds blur when skin stings and vision clouds, so muscle memory helps. For acid burns, flushing right away gives the best shot at reducing injury. Knowing the basics—where to find neutralizers, how to sweep up broken glass, what not to touch—strengthens quick thinking in a crisis.
Training sessions often feel repetitive, but each story shared comes from real mistakes. Hearing a coworker talk about an incident sticks better than reading a list of rules. Familiarity with emergency phone numbers and local protocols should not gather dust with time. I’ve noticed that reminders and open conversations about incidents remind everyone: no one is immune, and a lapse in attention can cost more than just a wasted afternoon. Openly reporting spills or near-misses, without fear, builds a safer routine for everyone.
I’ve learned to look out for colleagues, whether reminding someone to wear their gloves or helping them understand warning signs. Culture grows stronger when everyone expects—even demands—protective habits. Respect for hazardous materials begins with respect for your teammates’ safety as well as your own. Each of us brings something to the table: a new trick for storage, a shortcut for quick cleanup, or just the discipline to check twice before pouring.
Dealing with specialty chemicals like 3-(N,N-Dimethylaminopropyl)Aminopropyl methyl dimethoxysilane has never been about just tossing them on a shelf. Anyone who has opened a drum after a few months knows the trouble that comes from cutting corners. I recall being in a small lab years ago, watching as a colleague cracked open a jug of silane that hadn’t been sealed tight. The results weren’t dramatic—just a stubborn crust and a mess across the bench—but it sent all the wrong messages about respect for chemical storage.
This compound reacts badly with water in the air, transforming and sometimes producing byproducts that cause headaches during clean-up. Fold in health and safety, plus cost, and proper storage becomes something you can’t ignore.
The key issue isn’t just liquid containment; it’s moisture. Organosilanes do odd things in humid environments. Even a few drops of condensation spell trouble. Water triggers hydrolysis, gumming up the works and making drums hard to clean. You end up with less pure chemical, more downtime, and unnecessary costs. Folks who shuttle smaller samples between labs will instantly recognize the sticky residue and wasted product left behind by lax protocols.
Keeping this silane where it should be isn’t complicated, but it does ask for discipline. I’ve always kept it in that sweet spot: tightly closed in the original container, stowed away from sunlight and heavy traffic. Wide swings in temperature do more harm than many expect. That thermal cycling encourages condensation inside containers, drawing in moisture as things cool down.
Ideally, you want a cool, dry storeroom with reliable ventilation. Nothing high-tech required, but it pays to check humidity levels. Silanes don’t thrive above 30-40% relative humidity. I tell colleagues to watch out in summer months, especially when the air conditioner fails or doors get propped open. I’ve seen more than one batch ruined simply because someone stored containers near a loading dock exposed to humid air.
It’s tempting to use heavy-duty metal drums for everything, but organosilanes like this one often corrode certain metals after long contact. High-density polyethylene or polypropylene containers provide a better fit. I once lost half a batch because someone topped off a stainless-steel vat assuming it would be tough enough. Instead, we spent extra shifts cleaning out a strange gel that ruined sensors and filters.
Clear labeling and shelf-life tracking don’t always get the respect they deserve. Fresh stock performs better, and regular rotation keeps old product from causing surprise mishaps. I date every container I open and keep a log on a clipboard—boring, yes, but it stops mystery leaks and strange smells from building up in forgotten corners.
Aside from the hassle, improper storage creates safety risks. Skin contact brings burns and rashes, and vapors irritate the lungs. Wearing goggles and gloves isn’t just about liability—it actually prevents ruined weekends and time at the doctor. Proper storage doesn’t just protect business assets, but the people working shoulder-to-shoulder in the lab or warehouse. For a business, the costs are more than product loss. Faulty handling racks up downtime from cleaning, retraining, and patching up storage rooms.
Storing chemicals like 3-(N,N-Dimethylaminopropyl)Aminopropyl methyl dimethoxysilane is really about building a routine that respects risk and saves money over the long haul. It comes down to dry space, good containers, and simple routines. That approach won’t win awards, but it keeps operations humming and people healthy.
Ask anyone working with chemicals, pharmaceuticals, or food ingredients, and you’ll quickly hear the same stories. A product’s purity makes or breaks its usefulness. Picture a baker relying on sugar that contains trace contaminants. Even a fraction of something unwanted changes everything—the taste, the look, the health impact on customers.
Let’s walk through what purity actually means. In practice, it refers to how much of a product matches what it says on the label. If you’re dealing with sodium chloride, purity would measure how much is true salt and not other minerals or residues. Laboratory tests—like high-performance liquid chromatography or gas chromatography—pick up these differences down to the smallest percent. Many pharmaceutical labs set the bar at 99.0% or higher for their active ingredients, out of genuine concern for patient safety.
Details behind the digits really matter. Picture quality control in a hospital pharmacy. An impurity of even 0.1% might sound tiny, but over multiple uses or with sensitive patient groups, real harm can stack up. Regulations keep companies on their toes: the U.S. Pharmacopeia (USP) and European Pharmacopoeia each set strict definitions for “pharmaceutical grade” or “food grade.” For industrial chemicals, ASTM standards provide similar checks and balances.
True trust builds with transparency. Reputable suppliers publish detailed Certificates of Analysis with every batch. I’ve seen how a missing or ambiguous certificate can grind production to a halt. Teams can spend hours chasing down a missing data point. Clarity in reporting prevents expensive downtime and supports safe operations.
Supply chain issues in recent years have highlighted how vital these details stay. Without robust records, buyers run the risk of receiving off-spec material. This isn’t just a paperwork problem. Contaminated ingredients can force recalls, lead to lawsuits, and damage a company’s reputation that took years to build. Consistency in quality turns casual buyers into long-term customers, especially if they're dealing with high-stakes work like vaccines, electronics, or biomedical devices.
Money sometimes complicates the issue. Higher purity usually means more expensive processing and a steeper price. Cutting corners with a cheaper product might seem tempting, but hidden costs pop up down the line. Cleaning processes become tougher, waste goes up, and safety risks multiply. A decision based solely on price rarely turns out well in the long run. Smart companies weigh short-term expense against the value of reliability, clean documentation, and lower risk.
Science never really stops. Labs keep developing better tools to spot and remove impurities. Technology like ultra-high-resolution mass spectrometry exposes new trace contaminants that went unnoticed before. New regulations push everyone to improve, and customer demands set the pace.
I still remember a factory tour where the manager explained how even changing the water supply altered their end product’s purity and forced months of troubleshooting. It’s details like these that always stick—sometimes progress arrives from fixing the smallest slip-ups.
At the end of the day, purity specification isn’t just a line in a technical document. It protects people, businesses, and brands. Attention to these details keeps the world turning—safely, consistently, and with integrity.Manufacturers face tough decisions. Picking a resin or polymer isn’t just about function. Costs stack up, and the wrong fit causes headaches later. I’ve seen engineers frustrated because a simple oversight turned a small run into a batch of wasted supplies—dollars lost, trust shaken. Microcracks or failed bonds often trace back to mismatched chemistry. Most buyers want to know: will this product really work with what I have in mind? No one wants unplanned breakdowns just because two plastics decided not to “get along.”
Experience shows ABS, polycarbonate, and PET don’t react the same way to each additive. Some adhesives do their job on vinyl but barely grip nylon. People mixing new flame retardants into polypropylene blend for cable housing get the wrong idea about performance if they forget about compatibility. I remember a time when a batch of parts came out warped after switching to a cheaper resin. The product rep blamed storage, but it turned out the stabilizer didn’t align with the new base material’s chemistry.
Look out for published compatibility charts and real-world case studies. Reliable suppliers back up their claims with independent lab results. Uneven adhesion, color streaking, and material brittleness are red flags. The best industry partners share test data for various polymers, including mechanical and aging trials. This transparency builds confidence. Data points from sources like the American Chemical Society and the Society of Plastics Engineers show the scale of failed projects due to poor compatibility analysis. Companies lose millions each year correcting issues that a better up-front review could solve.
Every phone case, auto bumper, and food container faces a harsh world: heat, cold, cleaning products, time on a warehouse shelf. Compatibility determines if the final piece stands up or breaks down. Families expect a baby bottle to stay strong after repeated washes. Consumers want a car dashboard that doesn’t fade or crack after a summer in Texas. Choosing properly matched materials keeps warranties intact and complaints to a minimum. Poor choices mean tough reviews and damaged reputations.
Experienced engineers keep a shortlist of trusted suppliers and double-check new claims. They demand both raw compatibility data and user feedback from the field. Manufacturers should always run small trials before full production—some surprises don’t show up in the lab. Equipment makers and chemical formulators need open conversations about what works with what. This back-and-forth saves costs and gives peace of mind. Industry groups like ASTM International provide updated guidelines and set a bar for expected performance, thanks to years of collaborative work among experts.
People rarely think about the science behind common plastics, but every bottle cap and appliance part started with hard choices based on facts and testing, not just marketing claims. When labs and factories work together, final products last longer, draw fewer complaints, and keep end users happy. Compatibility means less waste, better performance, and reassurance that the product will do its job year after year.
| Names | |
| Preferred IUPAC name | 3-[3-(Dimethylamino)propylamino]propyl(dimethoxy)methylsilane |
| Other names |
Bis[3-(dimethylamino)propyl]methylsilane N,N-Dimethylaminopropylaminopropyl methyl dimethoxysilane 3-(Dimethylaminopropyl)aminopropylmethyl-dimethoxysilane 3-[2-(Dimethylamino)ethylamino]propylmethyldimethoxysilane |
| Pronunciation | /θri ɛn ɛn daɪˈmɛθəl-æmɪnoʊˈproʊpɪl æmɪnoʊˈproʊpɪl ˈmɛθəl daɪməˈθɒksiˌsaɪleɪn/ |
| Identifiers | |
| CAS Number | 13822-56-5 |
| 3D model (JSmol) | `load "CCN(CCCN)CCCO[Si](C)(OC)OC"` |
| Beilstein Reference | 3567994 |
| ChEBI | CHEBI:131549 |
| ChEMBL | CHEMBL571705 |
| ChemSpider | 2245534 |
| DrugBank | DB04353 |
| ECHA InfoCard | ECHA InfoCard: 100.244.234 |
| EC Number | 213-817-0 |
| Gmelin Reference | Gm 8974.1.3 |
| KEGG | C18697 |
| MeSH | C15H37N3O2Si |
| PubChem CID | 155095376 |
| RTECS number | UB2975000 |
| UNII | 1TT86KZ38N |
| UN number | 2735 |
| Properties | |
| Chemical formula | C10H26N2O2Si |
| Molar mass | 236.41 g/mol |
| Appearance | Colorless to yellowish transparent liquid |
| Odor | Amine-like |
| Density | 0.92 g/mL at 25 °C |
| Solubility in water | soluble |
| log P | 0.2 |
| Vapor pressure | 0.4 hPa (20 °C) |
| Acidity (pKa) | 10.18 |
| Basicity (pKb) | 6.3 |
| Refractive index (nD) | 1.427 |
| Viscosity | 10 cP |
| Dipole moment | 3.2182 D |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH⦵298) | -435.8 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P363, P501 |
| Flash point | > 96 °C |
| Autoignition temperature | 270 °C |
| LD50 (median dose) | LD50 (median dose): Oral, rat: >2000 mg/kg |
| NIOSH | GVW25024T0 |
| PEL (Permissible) | Not established |
| REL (Recommended) | Not Established |
| Related compounds | |
| Related compounds |
3-Aminopropyltriethoxysilane N-Phenylaminopropyltrimethoxysilane 3-(N-Ethylamino)propyltrimethoxysilane 3-(N,N-Dimethylamino)propyltrimethoxysilane 3-(N,N-Diethylamino)propyltrimethoxysilane N-(2-Aminoethyl)-3-aminopropyltrimethoxysilane |