
Scientists looking for ways to marry organic and inorganic chemistry have always kept an eye on silanes. In the 1960s, surface treatment needs in the plastics, rubber, and glass industries called for better coupling agents. Chemists didn’t rest on basic alkylsilanes; eventually, they searched for functional groups with higher reactivity. Adding a thiocyanate group to the silane backbone brought about a game-changer. At the time, trialkoxysilanes were already helping bridge surfaces, but the addition of the propyl and thiocyanato groups led to enhanced adhesion and chemical bonding, expanding the scope for silane technology in new material fields. Research communities took notice, testing and tweaking this compound to find reliable preparation methods and industry-grade purity. Over decades, this molecule found its place in the toolkit for building better composites, coatings, and elastomers.
3-Thiocyanatopropyltriethoxysilane, often abbreviated as TCPTES, enters the scene as a transparent, colorless to slightly yellow liquid. For those who work with glass fibers or reinforced plastics, its smell is hard to forget. Silanes like this don’t just glue things together; they modify surfaces for better grit, water resistance, and aging properties. You’ll spot this molecule in formulations for adhesives and rubbers where bond strength under stress makes all the difference. Companies label this product in drums or bottles, handling it as a specialty additive for multi-step industrial processes. Each batch carries a promise of consistency for tight production specs.
People in labs handle TCPTES with care, eyeing its boiling point around 285°C and a density a touch above one. Its refractive index sits near 1.44 at standard conditions. The triethoxysilyl group helps hydrolyze and cross-link with surfaces containing hydroxyl groups. If moisture hovers in the air, this compound reacts, forming silanols and releasing ethanol. The thiocyanate group isn’t just decorative—it’s known for boosting chemical reactivity, letting the molecule tie into sulfur-containing materials or participate in further modifications. Chemical stability holds firm under neutral pH, but acidic or basic conditions can trigger hydrolysis, urging careful storage and handling.
Product sheets usually list a purity of at least 97%. Labs check for water content under 0.5% and set specific limits on chloride and sulfur impurities. Most suppliers stamp the CAS number 34708-08-2 on the label. Drums carry UN shipping codes, and manufacturers print hazard pictograms alerting users to irritant risks. The Safety Data Sheet (SDS) jumps straight to handling directions. Storage conditions demand cool, dry, and airtight environments—no improvising with leaky containers or working in damp surroundings. The information on the label isn’t just for compliance—it points production managers to the safe and effective application of each lot.
A classic approach to synthesizing TCPTES starts with 3-chloropropyltriethoxysilane. Reacting this intermediate with potassium thiocyanate in a suitable solvent, such as acetone or a polar aprotic mix, pushes the reaction forward. After stirring or refluxing under controlled temperature, filtration removes potassium chloride byproduct. Distillation under vacuum cleans up the final product. Seasoned chemists keep a careful eye on the reaction’s progress, using thin-layer chromatography or gas chromatography for purity checks. Quality control takes batch-to-batch consistency seriously, looking out for trace contaminants that could throw off downstream applications.
TCPTES doesn’t stay on the shelf for long; its main role is reacting. Industrial chemists covalently bond it to surfaces with available silanol groups, like glass or silica. Mixing it in water or alcohol, often with a dash of acid as a catalyst, triggers hydrolysis. The resulting silanol species form robust Si-O-Si bridges with materials rich in hydroxyl groups. The thiocyanato functionality stands ready for secondary transformations. Under the right conditions, it undergoes nucleophilic addition or substitution—opening the door for custom surface attachments or further chemical elaborations. In composite making, it sets the stage for long-term durability and resistance to chemical attack.
TCPTES goes by a handful of other names in catalogs and technical papers: 3-Thiocyanatopropyltriethoxysilane, Propyltriethoxysilane, 3-thiocyanato-, Triethoxy(3-thiocyanatopropyl)silane, and sometimes even less formal trade names. Distributors branding the product for the construction, automotive, or electronics sector might add product line codes for easier tracking. Reading the fine print can spare a lot of confusion in order forms, especially when comparing global suppliers or cross-referencing technical info.
Anyone handling TCPTES in a plant or research setting needs gloves, goggles, and a well-ventilated space. This liquid irritates skin and eyes on contact. Some countries enforce exposure limits, so factory managers monitor air concentrations and ventilation rates. Good training goes a long way—a mistake with a silane can set off allergic reactions or respiratory issues. Spills call for sand or absorbent, never water, since hydrolysis can build pressure in closed containers. Fire risks stay low due to its high boiling point, but static discharge remains a threat in dry working environments. Waste management requires hazardous collection for incineration. Companies prefer closed-system transfers to minimize direct handling. Safety audits check labeling, storage, and first-aid gear for regulatory compliance.
In rubber manufacturing, TCPTES acts as a coupling agent, helping merge silica filler with polymer backbones, producing tires that last longer and grip better on wet roads. The glass industry treats fibers with this silane to improve the durability of reinforced plastics. Paint makers tap into its properties to bond pigments or fillers to resin matrices, crafting coatings that resist yellowing and peeling under sunlight. Some cable makers work with TCPTES for better insulation adhesion, reducing the risk of breakdown in challenging conditions. Materials science researchers keep testing it for new compatibilization challenges, like improving adhesion in composites made from reclaimed materials.
The research team that’s curious about new silane chemistries often turns to TCPTES for the molecule’s dual reactivity. Materials scientists experiment with loading nanoparticles, textiles, or ceramics to control surface wettability, barrier properties, or biodegradability. Developers continue to explore hybrid organic-inorganic materials, followed by rigorous mechanical and environmental testing. Studies look for optimal conditions—right down to pH, catalyst load, or reaction time—since minor tweaks can swing performance outcomes. Journals detail how changes in the silane’s structure tune compatibility between otherwise mismatched materials. Companies invest in pilot-scale trials before committing to full-scale production, always watching for patent conflicts or regulatory hurdles.
Toxicologists don’t take new silanes lightly. Dashboards of animal and cell culture studies review acute and chronic exposure effects. Short-term contact usually triggers mild to moderate skin and eye irritation, much like other trialkoxysilanes. Oral and dermal toxicity studies in rodents point to generally low systemic risk at typical industrial concentrations, though direct ingestion or prolonged skin contact can lead to more pronounced symptoms. Workplace health specialists keep an eye out for any reports of allergic reactions or asthma-like symptoms among operators handling this class of silanes over long periods. Environmental researchers stress proper disposal because hydrolysis by-products or unreacted silane can pose risks to aquatic habitats. Regulatory agencies in the EU and US have drafted guidance for manufacturer disclosure on safety data sheets, helping downstream users take preventive steps.
As industries push for greener, tougher materials, TCPTES stands poised for wider adoption. Companies investing in lightweight composites for electric vehicles target every molecule that can add performance without extra mass. The construction sector seeks new ways to bond recycled glass or silica to modern matrix resins, meeting sustainability goals while boosting product lifespan. Researchers in biomaterials look into tweaking the molecule, tailoring surface reactivity for medical implants, or controlled drug release. The next breakthroughs may involve process intensification—lowering energy use during synthesis or finding ways to capture and reuse by-products. Meanwhile, digitized material platforms crunch big data on substances like TCPTES, hinting at even smarter recipes for tomorrow’s high-performing, eco-friendlier products.
My first introduction to 3-Thiocyanatopropyltriethoxysilane happened in a tiny materials lab on the edge of our industrial zone. One of the chemists rubbed her hands together, grinning at a beaker of cloudy liquid. “This is the secret sauce for making things stick,” she told me. She wasn’t kidding. This molecule, with its silane backbone and thiocyanate group, finds its way into a surprising range of products—especially where you want something built to last.
Try bonding rubber to metal in a car engine mount. Without the right coupling, vibrations send things rattling and falling apart before the warranty expires. The silane structure latches onto metal or glass surfaces, while the organic side gets cozy with rubbers or plastics. That bridge helps manufacturers build composite materials and structural adhesives that meet tough standards. These are applications where cutting corners can mean expensive recalls or even danger on the road.
Walk onto any construction site and you’ll see concrete, glass, and steel, all going together in ways they never used to. 3-Thiocyanatopropyltriethoxysilane comes into play here, too. Silanes have a reputation for helping glass fibers bond to resins in fiber-reinforced composites. This single molecule tightens the hold between the two materials, so what you end up with resists cracking—even after years of bad weather.
A big reason for using this particular silane over more common types is that thiocyanate group. It boosts resistance to moisture and aggressive chemicals. Not everyone thinks about what happens when water sneaks into bridge supports or building panels, but over time, corrosion and breakdown can become dangerous problems. Well-designed surface treatments, using this silane, buy years of reliable performance.
Technology marches forward—and so do problems with static buildup, corrosion, and electrical breakdowns in sensitive equipment. In electronics manufacturing, 3-Thiocyanatopropyltriethoxysilane helps insulate tiny connectors, seal circuit boards, and prevent surface charge buildup. Ask any engineer about electrical failures and you’ll hear stories of missed deadlines, lost revenue, and a race to trace the source. A dependable silane treatment reduces these headaches.
Getting the best results with this silane means careful application. Surface prep remains a big struggle in many industries. Skipping cleaning steps, working with contaminated surfaces, or rushing the curing process leads to weak bonds and wasted materials. Chemical expertise makes a world of difference—plant managers and production supervisors know that close attention on the factory floor saves millions over time.
Regulation is tightening, especially in Europe and North America. Safe handling and environmental impact come into play, since silanes, including this one, can introduce toxic by-products if not managed carefully. Training and monitoring reduce risks for workers and stop spills from reaching water or soil.
Most of the change in this field happens through collaboration. Chemists, engineers, and frontline workers talk about what’s failing, what’s working, and what could use an upgrade. Technical knowledge gets passed down, from an old-timer in the adhesives shop to a young materials scientist, and the industry inches forward.
Look at any new bridge, wind turbine, or electric car component, and there’s a strong chance a silane treatment holds the invisible backbone together. With the right process, the right people, and some real-world wisdom, 3-Thiocyanatopropyltriethoxysilane earns its keep in ways you probably won’t see—unless something fails.
Working around chemicals like 3-Thiocyanatopropyltriethoxysilane isn’t just another day at the plant. This compound, used to modify surfaces or link organic and inorganic materials, carries its own set of rules if you want trouble kept at bay. Years spent in industrial labs taught me one thing: ignore a material’s needs, and you’ll get more than you bargained for—spills, spoiled batches, or worse, health scares that stay with you.
Expose this silane to moisture in the air and it starts reacting before you planned. Every chemist I know has seen a container that wasn’t tightly sealed, only to find the product degraded or the storage area contaminated. The right practice involves choosing a storage spot out of direct sunlight, away from heat sources, and especially away from water or humidity. A dry, cool environment not only preserves the silane but also protects the crew.
Silane fumes aren’t something to breathe in—even small leaks can escape detection if proper ventilation is an afterthought. Older storage rooms often lack the right exhaust systems, so many sites have shifted to improved air circulation. A lesson learned the hard way on several occasions.
Glass won’t cut it if you expect to store large volumes over time. Industry trusts tightly sealed metal drums or high-density polyethylene containers, and there’s a simple reason: these materials don’t get eaten away by silane, and you can count on them to keep out moisture. A friend once used the wrong plastic, and the result was a sticky mess that forced a shutdown for cleanup. No one forgets that sort of costly mistake.
Once, a mislabeled drum nearly led to a dangerous mix-up during production. Every drum should have clear, legible labels including the chemical’s name, hazard symbols, and handling instructions. It only takes a careless moment for someone to grab the wrong container in a busy warehouse.
This silane doesn’t get along well with strong acids, bases, or oxidizers. Storing incompatible chemicals together invites reactions you’d rather read about than experience. I’ve seen seasoned technicians double-check segregation charts before adding anything new to the inventory—habits like these stem from real scares and near misses.
Personal protective equipment isn’t optional. Splash-resistant goggles, chemical-resistant gloves, and lab coats keep skin and eyes safe from accidental contact. Stories circle in every lab about burns from neglecting safety gear, reminding everyone that procedures exist for a reason.
Preparedness plays a huge role in limiting damage. Spills must be mopped up before they seep into cracks or drains. Absorbent pads rated for organic liquid spills work best. Workers know to clear the area, ventilate the space, and follow up with a thorough surface decontamination. Quick response limits downtime and protects team well-being, lessons drilled into every onboarding session.
If contact happens, emergency eyewash stations and showers should be within easy reach. Anyone exposed flushes the affected area immediately. You can’t gamble with time when dealing with chemicals that can sneak through skin or lungs.
Most mishaps come down to skipped steps or overlooked details. Implementing strict handling and storage procedures isn’t about bogging down workflow; it’s about sending everyone home safely and keeping material in top condition. Training people on these practices, reinforcing the basics, and updating protocols when problems surface—these simple measures make a real difference in any facility working with 3-Thiocyanatopropyltriethoxysilane.
Working with chemicals like 3-Thiocyanatopropyltriethoxysilane feels routine for many scientists and lab techs. Over the years, I’ve watched new colleagues rush through a procedure, only to learn the hard way that no compound is “basic” once safety corners get cut. Handling this silane brings some real risks. Touching the substance can quickly irritate skin and eyes. Inhaling its fumes often leads to coughing, headaches, and a sore throat, so ignoring fume hood advice eventually bites back. Forgetting to wear goggles or gloves—people think their skin is tough—can end a shift at the emergency room instead of at a desk.
Ethoxysilane portions create extra worry about flammability. I remember a small splash landed on a hot plate in a university lab. There wasn’t a dramatic fireball, but the sharp smell and little blue flash made us all back up. This compound does not mix well with moisture, either. It reacts with water in the air, producing ethanol and irritating gases. Forgetting to seal the container or working in an unventilated space always risks greater exposure, and repeated mistakes often attract the attention of a safety officer—sometimes with disciplinary action.
Anyone used to working with organic molecules pays extra attention to getting even small droplets off their skin fast. Studies show compounds like 3-Thiocyanatopropyltriethoxysilane can penetrate skin, so even a little contact risks harm. Repeated exposure, especially in poorly managed labs or warehouses, brings up worries about long-term health—respiratory irritation, sensitization, and potential for chronic issues.
People trust gear more than training, but I’ve learned both matter equally. Thick nitrile gloves stand up to the silane better than latex gloves. Splash-proof goggles and good ventilation cut down nearly every exposure problem I’ve seen. The fume hood isn’t a “maybe” with this stuff; it's essential. Keeping an eyewash station and a clear spill kit nearby made a difference the moment someone knocked over a beaker. Training new staff to understand why each step matters—like double-checking for cracks in containers, and labeling every bottle—wins out against any top-down safety rulebook.
Locking away the bottle in a dry, cool cabinet backed by a chemical inventory helps keep things simple. I’ve found that storing incompatible materials—strong acids, bases, or oxidizers—separately helps avoid surprise reactions. Disposing of waste with the support of certified hazardous waste handlers prevents the temptation to pour leftovers down the drain. Many companies now track chemical inventories digitally, keeping tabs on small leaks and inventory errors before they turn into safety nightmares.
I’ve learned that healthy skepticism about any new compound always beats complacency. 3-Thiocyanatopropyltriethoxysilane asks for respect, not fear. Teamwork between experienced lab techs and eager learners made our lab safer—reminding each other about old lessons and pushing for better safety plans. Building these habits, not just relying on warning labels and data sheets, proved the most important line of defense.
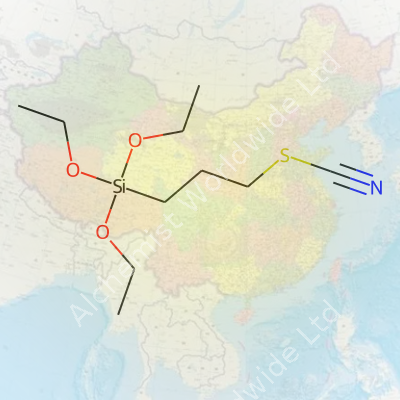
Chemical structures tell us more than any data sheet ever could. 3-Thiocyanatopropyltriethoxysilane holds the formula C10H21NO3SSi. Its backbone features a three-carbon alkyl chain. On one end sits a triethoxysilane group — think of it as three ethoxy groups (–OCH2CH3) hanging onto a single silicon atom. On the opposite end, you find a thiocyanate group (–SCN): that’s a sulfur atom bonded to a carbon, and the carbon presses up against a nitrogen. Written out, the structure looks like this: (C2H5O)3Si–(CH2)3–SCN.
Seeing a molecule’s bones helps predict its character. In the lab, I’ve seen thiocyanate and silane groups used separately. Bringing them together into one structure amps up their usefulness for surface chemistry. The silicon core gives it a leg up, since surfaces like glass or metal almost beg for that silane hook. The propyl chain gives the whole thing some stretch, plenty of flex to reach from the hard surface into whatever’s nearby. On the far end, the –SCN adds a reactive spot you can use for further chemical twists.
People sometimes overlook just how much a molecule’s setup changes its job performance. The triethoxysilane group loves to grab onto surfaces rich in hydroxyl groups. Ask anyone who’s tried to get paint or rubber to stick to glass — silanes move the job from impossible to routine. The –SCN group is less common in silanes, but it brings its sharp edge to polymer science. Here, we’re talking about a bridge builder between organic and inorganic worlds.
Let’s face it, no surface treatment works properly if the anchoring part gets left out. Many times, fixing surface adhesion is the difference between a high-performance sealant and a brittle, crumbling mess. 3-Thiocyanatopropyltriethoxysilane gives chemists a handle both for holding onto the surface and for connecting with latex, polyurethane, or epoxy systems. I’ve mixed batches with similar silanes before, and the difference jumps out after just a few months of use — better water resistance, lower risk of cracks, easier handling in both automated and manual setups.
The molecule’s not free of trouble. The –SCN group brings toxicity worries. Good ventilation, gloves, and clear storage protocols become non-negotiable. I’ve had a spill before; luckily it was caught in a fume hood, but nobody wants to test their luck with thiocyanate again. Wiser usage calls for substitution studies or clear end-of-life collection practices so it doesn’t find its way into water systems.
To get the most out of it, matching the silane’s strengths with the end product’s weakness works best. In composite manufacturing, people sometimes flood surfaces with generic primers and hope for the best. A tailored approach using 3-Thiocyanatopropyltriethoxysilane only where high chemical resistance and firm bonding truly matter pays off. This comes through training, which in my experience goes further than any data sheet or manual — real-world handling, practical workshops, and direct troubleshooting.
Future steps should focus on safer alternatives without losing the anchoring power this structure brings. Tighter regulation, especially on end-user awareness, can help shrink its environmental footprint. Bringing together hands-on training, open sharing of application results, and more transparency about what’s inside industrial chemicals could make the risks as clear as their rewards.
Surfaces play a bigger part in our lives than most people realize. The strength of a composite, the longevity of a plastic, and how clean a window stays in the rain often come down to chemistry at the very edge of the material. A mouthful like 3-thiocyanatopropyltriethoxysilane (known in labs as 3-TCPTES) offers something special. This compound manages to anchor itself to glass, metal oxides, or mineral fillers, and then it reaches out a chemical handshake to a polymer or resin.
Many organosilanes promise to bridge organic and inorganic worlds, but 3-TCPTES swaps out the typical amine or epoxy group for a thiocyanato group. That switch gives manufacturers a new handle for making strong, lasting connections between dissimilar pieces. The thiocyanato end shows useful reactivity with certain types of rubbers and engineering plastics, like those found in cables, car tires, or specialty adhesives.
My own work with surface treatments started in a research lab surrounded by glassware, solvents, and a frustrating number of sticky residues. We looked for ways to get silanes to stick just right, forming a thin layer that would stay put under pressure, heat, and moisture. 3-TCPTES won our attention because it handled water better than you might expect. Its triethoxysilane tail hydrolyzes—reacts with a touch of water—forming silanol groups that clutch to glass or silica with real tenacity.
This process creates a “primer” layer. On fiberglass or mineral powders, this primer lets the surface lock onto rubber or plastic. You only need a tiny amount—if too much builds up, you get bubbles or weak patches. Creating a good interface starts with careful dosages, sometimes a dilute solution in ethanol or another solvent. The coated filler or fiber, allowed to dry and cure, acts like rebar in concrete, only at the molecular level.
Let’s say you’re making a rubber gasket for a chemical pump. Chemicals attack the connection points over time; water, acids, or solvents try to wedge apart the filler and the rubber. A surface modified with 3-TCPTES resists this attack better. The organosilane connects tightly to both materials, making it harder for chemicals or heat to pry them apart. This extends product life, which means fewer repairs, less waste, and confidence that your equipment won’t leak when it matters.
In electronics, the story gets even more interesting. Glass fibers used in printed circuit boards or sensor housings handle heat and electrical stress. If the surface hasn’t been treated, humidity and movement work tiny gaps into the structure over time. In my experience, samples pre-treated with thiocyanate-functional silanes survive thermal cycling that leaves untreated samples delaminated or shorted out.
Safe handling and use require attention. The chemistry is robust, but mistakes happen if the prep isn’t right. Silanes should be stored dry and applied in well-ventilated environments. Overexposure can irritate skin and lungs, something I learned the hard way while cleaning up a spill during an overnight test. Training and ventilation matter, and so do gloves the right thickness for organic liquids.
On the sustainability front, there’s no shortcut. 3-TCPTES still comes from petrochemicals. Creating greener options will need new routes or recycling streams. What matters today is using the material wisely, matching the right silane to the right job, and never overapplying just to be “safe.” That’s how cost, safety, and performance line up to make real impacts for industry and for the people depending on these products every day.
| Names | |
| Preferred IUPAC name | 3-thiocyanatopropyl(triethoxy)silane |
| Other names |
3-Thiocyanatopropyltriethoxysilane 3-(Triethoxysilyl)propyl thiocyanate 3-Thiocyanatopropyltriethoxysilane, 95% Triethoxy(3-thiocyanatopropyl)silane 3-(Triethoxysilyl)propyl isothiocyanate Silane, triethoxy(3-thiocyanatopropyl)- |
| Pronunciation | /ˌθaɪ.oʊ.saɪˌæn.ə.toʊˈproʊ.pɪlˌtraɪˌɛθ.ɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | CAS Number: 34708-08-2 |
| 3D model (JSmol) | `3D (JSmol)` string for **3-Thiocyanatopropyltriethoxysilane**: ``` CCO[Si](CCCSC#N)(OCC)OCC ``` |
| Beilstein Reference | 1711518 |
| ChEBI | CHEBI:87143 |
| ChEMBL | CHEMBL4161709 |
| ChemSpider | 64836 |
| DrugBank | DB15262 |
| ECHA InfoCard | 01-000-001969 |
| EC Number | 220-949-4 |
| Gmelin Reference | 87887 |
| KEGG | C14303 |
| MeSH | D017207 |
| PubChem CID | 2734160 |
| RTECS number | UJ8585000 |
| UNII | 8J9R610B7X |
| UN number | 2810 |
| Properties | |
| Chemical formula | C10H21NO3SSi |
| Molar mass | 291.43 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Characteristic |
| Density | 1.05 g/mL at 25 °C (lit.) |
| Solubility in water | Soluble in water |
| log P | 2.1 |
| Vapor pressure | <0.1 hPa (20 °C) |
| Acidity (pKa) | 12.1 |
| Basicity (pKb) | 6.18 |
| Magnetic susceptibility (χ) | -67.0e-6 cm³/mol |
| Refractive index (nD) | 1.4700 |
| Viscosity | 3.5 mPa.s (20°C) |
| Dipole moment | 2.63 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 489.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07, Danger, H226, H302, H315, H319, H335, P210, P261, P280, P305+P351+P338, P337+P313 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H312, H332 |
| Precautionary statements | P264, P280, P301+P312, P302+P352, P305+P351+P338, P312 |
| NFPA 704 (fire diamond) | 3-2-1-W |
| Flash point | 93 °C |
| Autoignition temperature | 290 °C |
| Lethal dose or concentration | LD50 Oral Rat 1640 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 771 mg/kg |
| NIOSH | VV7525000 |
| REL (Recommended) | 10-30 mg/m³ |
| Related compounds | |
| Related compounds |
3-Cyanopropyltriethoxysilane 3-Mercaptopropyltriethoxysilane 3-Aminopropyltriethoxysilane 3-Chloropropyltriethoxysilane 3-Glycidoxypropyltriethoxysilane |