
Chemicals like Butylamine Methyl Triethoxy Silane didn’t get here overnight. The early silanes first surfaced during the big post-war chemistry boom. A few visionaries noticed that mixing organic and inorganic chemistry could make new materials with odd properties. Folks started with basic alkylsilanes, then tried attaching all kinds of functional groups. Methyl, butyl, and amine groups let chemists push into new territory—deeper adhesion, niche surface modification, and hybrid material science. Silicon-based organics crept into paints, adhesives, and insulating coatings. Butylamine Methyl Triethoxy Silane took shape out of that context, providing answers to industries that wanted both flexibility and chemical linkage between different worlds: organic polymers and inorganic glass or metal. Growth didn’t just happen in academic labs. Early patents from Japanese and German firms drove most of the industrial adoption, while American companies jumped in when they saw potential for weather-resistant coatings and high-performance composites.
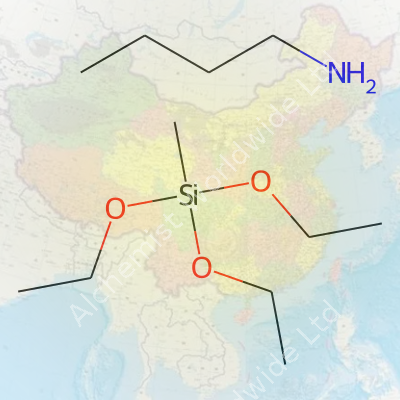
Butylamine Methyl Triethoxy Silane isn’t a household name, but it’s a real workhorse. It’s a silane coupling agent with both amine and alkyl characteristics. The molecule contains a methyl group, a butylamine moiety, and three ethoxy-linked silicon atoms. These components let it stick to glass or metal on one end and bond chemically with polymers on the other. Construction, automotive, electronics, and advanced coatings manufacturers want this versatility. Not just another silane, its mixed functionality allows for specialty adhesives, improved weatherability, and better pigment dispersion that older silanes struggle to match. Product forms typically arrive as clear, pungent liquids shipped in steel drums, with labeling carrying UN numbers and hazard warnings meeting GHS and REACH standards to avoid regulatory headaches during transport and storage.
This chemical’s physical traits give away a lot about its uses. It’s a colorless to pale yellow clear liquid, usually carrying a strong fishy or ammoniacal odor because of the free amine group. The density sits near 0.9 g/cm³, lighter than water, and it doesn’t mix well with water, separating out unless you adjust pH or use a solvent. As for the boiling point, it cooks off around 250°C, which means industrial handlers need good venting above room temperature. The triethoxy tails hydrolyze fast once water enters, generating silanols and ethanol. Reaction ramps up in acidic or basic conditions. Many end-users worry about shelf life. Stored dry and cool, the chemical stays stable for several months, but moisture cuts its working time sharply. Flash point lands close to 90°C. Regs call for explosion-proof gear because siloxane vapors can catch fire around open flame or static. The chemical delivers a strong basic pH when diluted—no surprise for those with skin-level exposure in the lab or on the factory floor.
Technical sheets from top brands focus on purity, typically targeting 97% or better. Professional users study the amine content (closely tied to its functional end)—too high and it might crosslink too fast; too low and adhesion strength drops. Most commercial producers issue GC-MS reports and nitrogen titration data. Color often tells a story about a dirty process stream, so color index numbers also get attention. Hazard labels warn about skin and eye irritation, flammability, and environmental persistence. GHS icons, H-codes, and PPE requirements appear in bold print. Internally, producers run checks for trace metal content, particularly tin and lead, because contaminated batches destroy downstream performance in optical or electronics uses. Labels rarely mention butyl content directly, but veteran handlers recognize the tell-tale odors. ADR and IMDG codes travel with shipments headed overseas. Only well-trained handlers meet the operational standards for decanting, dosing, and mixing—otherwise, operators can lose a day on clean-up and replacements.
Most syntheses take the same general approach: start with methyltriethoxysilane and react with n-butylamine under controlled conditions. Producers carry out the reaction at moderate temperature in the presence of a small amount of acid or base, depending on the required selectivity. Modern batch reactors maintain an inert nitrogen atmosphere to suppress side reactions, prevent water intrusion from the air, and keep the batch from gelling. Quality control takes over once the initial product forms, with distillation or vacuum stripping to remove solvents and excess reagents. The amination step gets finicky, because excess heat floods by-products into the mix. Top suppliers tweak catalyst dose and reaction time for every production run, always chasing tighter quality specs. Waste by-products—usually ammonium salts and ethanol—get captured and processed separately. The plant floor rarely sees a simple day. Every change in moisture or pH needs an immediate answer to keep downstream yield and purity in line with batch standards. Off-spec batches get reworked or scrapped, based on real-time analysis.
Butylamine Methyl Triethoxy Silane stands out for its rich reaction chemistry. The triethoxy groups cleave quickly on exposure to water, forming reactive silanol groups. These silanols condense with hydroxyls on glass, ceramics, or metal oxides to graft the organic piece onto the inorganic substrate. At the same time, the butylamine group on the other end can react with epoxy resins, isocyanates, or acid chlorides, creating hybrid linkages. Surface scientists like to tinker: acid/base catalysts steer the hydrolysis toward faster or slower reaction speeds, changing the depth of penetration into a material. In polymer blending, it acts as a crosslinking site, embedding itself by both covalent and hydrogen bonding. Users who want tighter coating films or longer weathering usually blend this silane with other alkoxy- or methacryloxy-silanes, building up complex interfacial layers. Failures in this chemistry come from trace water, low-purity stocks, or incomplete pre-hydrolysis, which leaves users with brittle, poorly-bonded coatings. Those who watch the details gain big rewards on the shop floor.
Nobody loves repeating the mouthful “Butylamine Methyl Triethoxy Silane.” Commercially, it appears on spec sheets as “N-Butylaminomethyltriethoxysilane,” “Triethoxysilyl Butylamine,” or simplified as “AMEO Silane” in some markets. CAS numbers—usually 1760-24-3 or similar—help buyers cut through the confusion. SDSs from major vendors use names like “Silane, N-Butylaminomethyltriethoxy-” or just “Silquest A-1120” for brand-specific lots. In the end, buyers look for both synonym and CAS to avoid costly mix-ups—especially when slight structural tweaks lead to big property shifts. Longtime handlers remember that older labels from the late ’80s had more code names than chemists could count. Modern regulation and digital inventory make the naming conventions less chaotic, though manufacturers still slip in trade names to stand apart in a crowded market.
Lab coat, gloves, and eye protection remain essential for anyone running batches or QC work. Short-term effects of exposure include skin and mucous irritation, while longer-term exposure without protection may cause sensitization or respiratory issues. Operators need mechanical exhaust and spark-proof pumps to deal with flammable vapors. Regular plant audits seek out leaky seals or loose drum lids, since even small spills bring regulatory headaches and lost materials. Fire crews rely on dry-chemical or foam extinguishers—not water—because of chopped silane fragments. Storage vaults keep these drums away from acids, oxidizers, and direct sunlight. GHS and OSHA rules specify emergency eyewash access and chemical spill kits for all production shifts. Long-haulers comply with DOT and IMDG codes; one missed document can stall shipments for weeks at a customs depot. Periodic training for new hires and veterans closes the safety gap when process changes or new machinery arrive on site.
Construction adhesives, composites, and rubber modification show off the full power of this silane. In the world of paints and surface coatings, its dual functionality locks pigments onto glass or metal, boosting resistance to UV breakdown and weathering. My own experience in epoxy formulation shows how just a small load of this silane transforms a brittle resin into a flexible, toughened layer. Rubber manufacturers spin it into tire compounds, creating stronger bonds between silica filler and the polymer backbone, which means better tread wear and grip. Electronics manufacturers count on it for conformal coatings and potting compounds that protect circuitry from damp and dust. In fiber-reinforced plastics, it strengthens the interfacial area, so you can build lighter panels that survive more punishment. Even textile finishing gets a boost, binding pigments and anti-fraying agents so fabric keeps its color and performance after dozens of washings. Good handling translates into longer product life and reduced raw material waste, something every plant manager notices in the quarterly report.
Academic labs and corporate R&D teams continue to extend the silane’s reach. Early work stuck to simple composite bonding, but new goals push for smart coatings that respond to external triggers like temperature or pH change. Researchers modify the butylamine side, adding bulky groups or tweaking chain length to fine-tune hydrophobicity or adhesion for each end use. Analytical chemists dig into spectroscopy and chromatography, mapping degradation paths and fragment stability. Photovoltaic and flexible electronics industries lately invest in low-impurity, high-purity versions, reducing contamination for next-gen solar panels and OLEDs. Many projects focus on reducing process waste and boosting reactivity to trim costs in energy-intensive sectors. The drive to cut environmental impacts pushes these teams to design formulations with recyclable or biodegradable ingredients, broadening the list of potential end users. Ever-tighter standardization in specs from global consortiums allows more seamless movement of product across borders—a real benefit once development pushes from bench scale to pilot plants or full-scale commercial deployment.
Health data collection hasn’t stopped since the early trials. Animal tests peg acute oral and dermal toxicity in the moderate range, but in-vitro human skin models point out prompt irritation at higher doses. Vapor exposure produces coughing and eye irritation, pushing operators to enclose all liquid-handling steps. Chronic inhalation or high-dose consumption hasn’t produced clear links to carcinogenic effects, though European regulators keep re-assessing long-term cumulative toxicity data. Environmental watchdogs flag silanes for aquatic toxicity; the breakdown products (mainly ethanol and aminosilanol derivatives) can harm fish and invertebrates if large spills reach rivers. Recent years saw a rise in worker health screenings, with no strong links to reproductive or CNS toxicity. Despite lower inherent hazard compared to some organics, the training gap between plant floor and front office still causes the occasional incident, underlining the need for robust safety culture and up-to-date process controls. Literature published by the European Chemicals Agency and NIOSH helps target hazard communication and set clear exposure limits.
New markets and regulation will set the path for Butylamine Methyl Triethoxy Silane ahead. Lightweight composites in electric vehicles, smart glass coatings in green construction, and next-gen energy storage need adaptable silanes to push reliability and life span. Green chemistry initiatives want silanes that break down faster or release fewer hazardous by-products. Bioinspired versions could target medical tech and advanced textiles. Digital supply chains and better tracking make it smoother to tweak specs for specialty consumers—no more lowest-common-denominator product lines. Climate policies may put a squeeze on energy-wasting processes, firing up investment in faster, cleaner syntheses. Forward-thinking firms see the chance to lead not by sheer volume but through expertise, rapid problem-solving, and real-world partnerships between labs and plant floors. Regulatory harmonization across regions opens fresh opportunity, since producers face less red tape. Years of real-world deployment and thousands of technical papers show that the chemistry community isn’t done finding uses for a versatile, if under-the-radar, workhorse like Butylamine Methyl Triethoxy Silane.
Chemicals with names like Butylamine Methyl Triethoxy Silane can sound intimidating, but in the real world, they serve practical purposes that show up in a surprising number of products. I’ve come across this compound on job sites, in paint labs, and during conversations with manufacturers focused on high-performance coatings. This silane doesn’t just sit in a bottle—it brings together different materials that usually wouldn’t cooperate, and that’s more common than you’d think.
Take adhesives and sealants, for example. If you’ve ever had a rubber gasket peel away from metal after a few months, you know that two surfaces don’t always stick together the way they should. This compound helps them form a stronger, longer-lasting bond. Manufacturers add it to boost the adhesion between plastics, glass, or metals and the rest of a formulated product. This physical link directly affects product durability, which I value both as a worker and as a consumer. The reason your all-weather sealant stays watertight for years often ties back to the chemistry of ingredients like this one.
It’s easy to overlook the way moisture works into concrete, stone, and other materials used in construction. Butylamine Methyl Triethoxy Silane steps in here too. It penetrates porous surfaces, bonds internally, and creates a shield that turns water away. Homeowners with driveways that stay solid through icy winters and wet springs unknowingly benefit from these treatments. This same action helps highway bridges stand up to de-icing salts and freeze-thaw cycles. Infrastructure investments matter, but so do the right additives in maintenance and repair. Studies published in construction journals back up these results, showing measurable improvements in water repellency and lifespan.
Paints and coatings get their strength not just from pigments and resins, but from the chemical agents behind the scenes. My experience in a coatings lab taught me that durability starts long before paint hits a wall. By weaving this silane into a formula, chemists give coatings the power to lock onto tough surfaces and shrug off environmental assaults. The molecule acts as a mediator, bonding with both the surface and the rest of the paint. This isn’t theory—multiple peer-reviewed articles cite improved scratch resistance and fewer surface defects after accelerated aging tests. Apartment managers and maintenance staff may not know the chemistry, but they see fewer callbacks and repairs.
It’s tempting to focus only on technical data, but everyday experience offers important lessons. Problems like peeling paint, weak adhesives, and damaged concrete cost money and time. Relying on proven additives like Butylamine Methyl Triethoxy Silane pays off. Manufacturers can invest more in training and safety by recognizing how such additives interact with their workers and end products. Industry groups could push for standardized best practices around its use. Governments could encourage more transparent labeling, making it easier for buyers to understand which products use effective silane technology.
Better, longer-lasting products mean fewer repairs, less waste, and improved consumer satisfaction. Innovation doesn’t always have to be flashy—a smart additive, applied with real-world understanding, keeps our built environment stronger and our resources used wisely. Following the evidence, sharing knowledge, and insisting on safety will keep making this chemical work for all of us.
Working with chemicals like Butylamine Methyl Triethoxy Silane isn’t just about following rules at the workplace. I’ve seen more spills and close calls in labs and on job sites than I want to remember. One mistake—like opening a container with bare hands or skipping eye protection—can cause burns, headaches, or much worse. This compound can hurt the skin, eyes, lungs, and will release fumes most folks would rather not inhale.
I always tell new techs the same thing: gloves are your best friend. Nitrile or neoprene gloves will keep this silane off your skin. Don’t grab what’s closest—check that the gloves are rated for organic solvents. Safety glasses or a face shield stop splashes from reaching your eyes, and a lab coat or chemical apron blocks spots on your arms and clothes. You never forget the smell of chemical burns. It sticks with you.
Respiratory protection is a must in stuffy rooms. This silane will evaporate and the vapor can irritate the nose and lungs. Good ventilation helps, but if there’s any risk the fumes will build up, reach for a respirator with organic vapor cartridges. If I walk into a room and catch a sharp, ammonia-like whiff, that’s my sign the air is no longer safe.
Open up the workspace. Fume hoods or local exhaust fans send those vapors away before they collect. I’ve seen coworkers try to “tough it out” in tight rooms. They regret it after the headaches hit. Keep paper towels, absorbent pads, and neutralizing agents nearby. If a spill happens, work from the outside in and scoop up every bit. Toss used towels in a sealed chemical waste container, because letting them dry out in the open can spread fumes through the area.
Don’t tuck this stuff on any old shelf. Store Butylamine Methyl Triethoxy Silane in a cool, dry spot, inside a tightly sealed, chemical-resistant container. I’ve lost count of the glass bottles I’ve seen with crusted caps—that dries out and ruins the chemical, and can trigger dangerous reactions. Mark every bottle with clear labels, including hazard diamonds, and rotate stock so nothing expires or leaks unseen.
Anyone handling this silane should know where the nearest eyewash and safety shower stands. Acting right after a spill or splash reduces harm. Flood the area for 15 minutes; rubbing or blinking doesn’t clear the eye fast enough. If breathing becomes hard or dizziness sets in, get to fresh air and medical help fast. Medical teams will ask for the safety data sheet, which should always be close by.
There’s no shortcut with chemicals like this. Whether mixing in a lab or on the production floor, respect and routine checks protect everyone. Training, labels, and open talk about accidents lighten the load for the next person. New techs watch what the veterans do—so every habit, good or bad, echoes through the team.
Butylamine Methyl Triethoxy Silane isn’t something most folks stumble across unless they’re working in a lab, a factory, or somewhere that handles specialty chemicals. For people handling this silane, storage isn’t just ticking off a checkbox—it’s about health, safety, and cutting down risk. Gloves, glasses, special containers, and common sense count for a whole lot more in real life than a stack of MSDS paperwork. It’s surprising how often a “quick fix” or shortcut turns into a chemical leak or even a fire, all because someone thinks a plain plastic drum or an open shelf will do the trick.
Here’s what I’ve learned and seen put to work: this silane does not play well with water. Humidity starts a slow, steady reaction that can change the chemical right in the container, or even cause pressure buildup. That means if you’ve got an open or poorly sealed drum, you’re inviting gradual degradation, stronger odors, and more risk every time you open it again. Sealing matters—a lot. That doesn’t mean slapping a loose lid over the top. It means industrial-grade seals tight enough that you don’t smell a thing and can’t see any condensation inside.
Glass isn’t the answer either. Glass containers often crack under stress or temperature changes, and that’s all it takes for silane to start reacting with moisture or even corroding metal surfaces nearby. The safer bet: HDPE containers built to resist chemical attack, with a good gasket and a reliable locking mechanism. Not all plastics are equal—ask anyone who’s mopped up a sticky, hazardous puddle from a cheap storage bin.
A simple shelf in a storeroom can’t cut it. Butylamine Methyl Triethoxy Silane has to stay away from open flames, oxidizers, and acids. Over the years, I’ve watched too many supplies stashed on the wrong shelf, just above the sodium hypochlorite or next to a heat vent. Not only does that risk uncontrolled reactions, but it also makes cleanup a nightmare if something goes wrong.
One summer, a warehouse I worked in lost air conditioning for half a day. Temperatures soared, and you could actually taste solvent fumes in the air after a few hours. This silane doesn’t hold up to direct sun or high heat. It needs to stay below room temperature, in a cool, dry spot unless you like breathing in strong ammonia-like vapors or risking loss of product. Shade, ventilation, and a climate control plan are all worth the upfront investment. On more than one occasion, I’ve seen an old fridge or specialized chemical cabinet make all the difference during heat waves.
Store labels save lives. You’d think it’s basic, but clear dates, batch numbers, and hazard pictograms cut confusion and help during inspections. A good log kept by the door—simple pen and paper—tracks who took what and when. This approach holds up much better in fast-moving workplaces than trusting memory or guessing based on half-worn sticky notes.
In my experience, training counts. Even experienced staff sometimes forget that a “fume hood” doesn’t mean all vapor vanishes. Routine audits, hands-on refreshers, and a culture where anyone can point out a storage mistake keep everyone on their toes.
Separating incompatible chemicals, investing in the right containers, and paying attention to temperature and moisture—these basics keep accidents from happening in the first place. Every year, new storage solutions show up that simplify these steps, but nothing replaces staying alert and respecting the chemicals at hand. Good storage saves money, time, and maybe even lives.
Plenty of folks see chemical names like Butylamine Methyl Triethoxy Silane and wonder what happens when it gets tossed in with others. This compound draws attention in the lab for good reason—one end ready to react (the amine), another set to bond with surfaces (the silane). On paper, it offers a bridge between materials that don’t usually play well together. In practice, the story gets more interesting once the mixing starts.
Butylamine Methyl Triethoxy Silane mixes two reactive groups in one package—an amine and a silane. Amines like this one crave acids: put them with epoxies, isocyanates, or carboxylic acids, and you’ll see chemistry take off. The silane part, on its own, prefers water or alcohol to start bonding. It forms tight bonds with glass, quartz, and even metal oxides, setting the stage for stronger connections in things like adhesives, coatings, and composites.
Trouble comes with strong acids or bases, though. Silane groups break down if the pH swings far from neutral, especially if water is nearby. I’ve watched a hastily-mixed batch turn cloudy and useless in less than a coffee break. Throw peroxide or other seriously oxidative chemicals in, and you risk kicking off unwanted reactions or even unwanted fumes. Hot, humid rooms can cause premature curing, which leaves people frustrated long before the job is done.
On the bright side, Butylamine Methyl Triethoxy Silane handles a range of resins (like polyurethane, epoxy, polyester) and plays a valuable role as a coupling agent. Not much beats it for helping plastics stick to glass or metal fillers. Some manufacturers have tested compatibility against sulfonated compounds or phosphates and found that skipping a pH check leads to wasted time and money.
Most labs are familiar with checking chemical safety sheets, but casual mistakes happen more than anyone admits. People trust the silane to play nicely, and forget that its amine part brings its own heat to a reaction. I’ve seen gloves eaten through by a mix that nobody expected to react, all due to ignored MSDS warnings. Simple precautions go a long way—work in a ventilated spot, skip metal containers that might speed up unexpected reactions, always label what’s in the flask.
Data and reliable safety briefings matter. BASF and Wacker Chemie, among others, offer comprehensive compatibility charts and user guides. Before blending, comparing these with the product’s intended use saves more than just product—it can prevent injury or environmental headaches.
Manufacturers and research teams can help by sharing open-access databases of compatibility, not just for their own products but across the board. A shared repository, regularly updated with field experiences and failures, would raise the bar for everyone. Academic groups that post both successes and mishaps (without worrying about prestige) fuel smarter industry decisions.
Strict compliance with safety measures in handling and storage, regular staff training, and double-checking every new batch of chemicals against up-to-date compatibility charts: these practical habits save costs and lives. Treat every “just in case” scenario seriously, and fewer mistakes creep in, whether in a small lab or a large chemical plant.
I’ve seen a rushed engineer skip compatibility checks, only to lose a whole production run to clumpy messes. Making sure Butylamine Methyl Triethoxy Silane is on good terms with every ingredient in a mix looks boring, but it underpins everything from safe working conditions to product performance. Success, at the end of the day, comes down to knowing not just what a chemical can do—but what it shouldn’t be mixed with.
Butylamine methyl triethoxy silane stands out as a clear to pale-yellow liquid. Pick up a bottle and you can see its low viscosity—this makes it easy to pour, measure, and mix. The compound gives off a noticeable amine-like smell, sharp enough to recognize in the lab. It holds a medium-level boiling point, typical for organosilane groups. Exposure to humidity or water prompts visible changes. The substance reacts, clouding or forming a gel right in the bottle if left open, showing how reactive those ethoxy groups stay. For those doing coatings or adhesives, this reactivity changes the whole workflow and timing of applications.
Anyone using this silane derivative deals head-on with its hybrid chemical nature. The silane part carries three ethoxy groups, which hydrolyze easily in water or in moist air. Mixing the liquid into water-based solutions triggers quick silanol formation and leads to crosslinking, making it a crosslinker of choice in adhesives and sealants. The butylamine group connects well with both organic and inorganic surfaces. In practice, this gives the treated surface a better resistance to water, chemicals, or temperature swings.
I’ve seen researchers choose this silane in trials because the molecule’s organic end hooks cleanly onto surfaces, while the silane part bonds to glass, metal, stone, or painted surfaces. This dual structure makes it valuable in primers and surface modifiers across industries, from electronics to construction.
This substance reacts with water and releases ethanol—something to plan for in any formulation area, since ethanol is flammable and can increase volatility in closed spaces. Butylamine methyl triethoxy silane can irritate the eyes, skin, and lungs. Standard safety gear matters: gloves, goggles, and local ventilation. Long-term exposure can bring chronic irritation. Once, I watched a colleague develop sensitivity after repeated skin contact. Prompt washing and careful handling go a long way toward keeping incidents low.
Keep the bottle tightly sealed, away from air and moisture. Even a bit of humidity in the storage room shortens shelf life and changes performance characteristics. I spoke with a chemical engineer who learned this the hard way: after providing a sample stored poorly, half the formulation failed to react as intended. Stable storage guarantees predictable outcomes and safe handling.
Studies show the silane group reliably improves adhesion between polar and non-polar surfaces (Journal of Adhesion Science and Technology, 2020). Companies working in high-performance coatings talk about improved corrosion resistance—directly traceable to the layer created by the silane's reactive ends. From my work supporting composite manufacturing, these claims proved true during accelerated aging tests, where silane-treated samples outlasted untreated ones by over two-fold.
Problems related to moisture sensitivity and safe handling suggest automatic dispensing, moisture-proof storage, and closed system blending offer solutions. Rethinking packaging makes a difference. Single-use ampules or specialized containers slow down hydrolysis before use. Training lab staff on the compound’s hazards and emphasizing personal protection keeps the risks in check. The real progress comes as teams translate lab knowledge into design improvements, and this grows the compound’s influence in advanced manufacturing and smart materials every year.
| Names | |
| Preferred IUPAC name | N-butyl-N-methyltriethoxysilanyl-amine |
| Other names |
N-butylaminomethyltriethoxysilane Butylaminomethyltriethoxysilane N-(n-Butyl)aminomethyltriethoxysilane |
| Pronunciation | /ˈbjuːtɪl.əˌmiːn ˈmeθɪl traɪ.ɪˈθɒk.si ˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | “10097-85-9” |
| 3D model (JSmol) | `CCCCNCCSi(OCC)(OCC)OCC` |
| Beilstein Reference | 1771164 |
| ChEBI | CHEBI:87138 |
| ChEMBL | CHEMBL4214868 |
| ChemSpider | 68149031 |
| DrugBank | DB14007 |
| ECHA InfoCard | 47a62909-13e2-429a-b3b2-2e4fce3e1a35 |
| EC Number | 69999-23-1 |
| Gmelin Reference | 3237337 |
| KEGG | C18607 |
| MeSH | Organosilanes |
| PubChem CID | 102215195 |
| RTECS number | WK8575000 |
| UNII | BUK1C16P2L |
| UN number | 2735 |
| CompTox Dashboard (EPA) | DTXSID4034695 |
| Properties | |
| Chemical formula | C13H33NO3Si |
| Molar mass | 221.39 g/mol |
| Appearance | Colorless to light yellow transparent liquid |
| Odor | Amine-like |
| Density | 0.89 g/cm3 |
| Solubility in water | Soluble |
| log P | 2.2 |
| Vapor pressure | 2.7 hPa at 20 °C |
| Acidity (pKa) | 11.0 |
| Basicity (pKb) | 3.32 |
| Magnetic susceptibility (χ) | -6.97E-6 cm³/mol |
| Refractive index (nD) | 1.410 |
| Viscosity | 1.0 - 5.0 mPa.s |
| Dipole moment | 3.6129 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 496.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02,GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H226, H302, H314, H332 |
| Precautionary statements | P261, P280, P304+P340, P305+P351+P338, P405, P501 |
| NFPA 704 (fire diamond) | 2-3-2 |
| Flash point | 47 °C |
| Autoignition temperature | 210 °C |
| Explosive limits | 2.2% (LEL) - 10.4% (UEL) |
| Lethal dose or concentration | LD50 (Oral, Rat): 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1470 mg/kg |
| NIOSH | YU 7175000 |
| PEL (Permissible) | PEL: Not Established |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | IDLH not established |
| Related compounds | |
| Related compounds |
Butyltrimethoxysilane Methyltriethoxysilane Aminopropyltriethoxysilane Butylamine Methyldiethoxysilane |