
People carving paths in materials chemistry found themselves needing solutions that could push silicon’s role further—not just in chips and circuits, but in smart coatings, fine-tuned surfaces, and resilient bonding agents. Back in the mid-20th century, researchers started working with functionalized silanes, including derivatives packing aryl groups on the silicon core. The discovery of diphenyldiethoxysilane traces back to this period of restless lab work. Scientists built the molecule with two bulky phenyl rings and two ethoxy groups. Researchers kept their sights set on improving hydrolytic stability and adhesion in complex environments, especially as the coatings industry began chasing more durable, chemically resistant compounds for automotive, electronics, and aerospace work. Diphenyldiethoxysilane gradually established itself as a building block—part innovation, part necessity—for new generations of silicon-based polymers.
Manufacturers and materials scientists know diphenyldiethoxysilane as an organosilicon compound used in specialty polymer production, surface treatment, and as a coupling agent. It has served countless bench experiments and industrial processes aiming to graft organic groups onto silicon frameworks or protect surfaces from chemical attack. With its measured volatility and predictable hydrolysis, operators can manipulate it with more confidence than some more aggressive chlorosilanes. The two phenyl groups on the silicon atom help provide unique resistance to UV degradation and oxidative processes, making it a preferred option for labs and factories seeking better performance from treated substrates and composite materials.
Diphenyldiethoxysilane appears as a clear or pale-yellow liquid, usually with a faint aromatic odor. Its molecular weight hovers around 286 g/mol. The boiling point, reaching in the region of 310°C, signals a level of thermal stability that stands out—giving it legs in high-temperature manufacturing. Its density comes in slightly heavier than water at roughly 1.03–1.05 g/cm³, so it’s manageable for most lab setups. It dissolves in common organic solvents—ethers, chlorinated hydrocarbons, and benzene family compounds—offering plenty of room for formulation flexibility. The compound handles moisture with a degree of caution, as it hydrolyzes to silanols and ethanol, a trait shared with other alkoxysilanes. Chemically, it treads the line between reactivity and robustness, which makes it a reliable candidate in controlled transformations.
Producers typically list diphenyldiethoxysilane with purities above 97%, aiming to accommodate research-grade and industrial protocols alike. Its CAS number, 2555-98-6, stays fixed on safety data sheets and drum labels. Handling requires gloves, goggles, and ventilation—ethyl silanes rarely cause trouble in small volume, but commercial scale-ups demand respect. Export and shipping paperwork usually feature UN codes covering flammable liquids, just in case, though its flash point lands above many run-of-the-mill solvents. Labels also touch on precautions: keeping containers sealed, avoiding prolonged sun exposure, and handling spills without water due to potential hydrolysis.
In my early days shadowing at a specialty chemicals plant, I followed the stepwise preparation of diphenyldiethoxysilane, which boils down to a Grignard reaction. Chemists began with diphenyldichlorosilane, treating it with an excess of ethanol in the presence of a base or sometimes just careful temperature control, swapping out the chlorine atoms for ethoxy groups. The process gives off hydrogen chloride as a byproduct. Strict temperature and moisture controls pay dividends here—ethanol and HCl create a tough environment for glassware, so continuous monitoring and inert atmospheres keep the batch on track. Once purified by distillation under reduced pressure, the product’s quality shows in its clarity and the absence of persistent halide odor, a sign the reaction reached full conversion.
Chemists experiment with diphenyldiethoxysilane as a silicon source for crosslinked networks and specialized coatings. On contact with trace water and acids or bases, it hydrolyzes, forming silanols and ethanol, and as condensation kicks in, complex siloxane bonds grow—ideal for durable, water-resistant polymers. The molecule’s two phenyl rings lend steric protection, so reaction rates shift with catalyst choices and temperature. In more advanced synthesis, researchers swap one or both ethoxy groups out by transetherification or alkoxylation, or tether further organic substituents to customize downstream properties. For electronic-grade work, ultra-pure product undergoes repeated distillation and scrubbing, reducing trace metals that could upset semiconductor grades.
In catalogs and procurement portals, diphenyldiethoxysilane shows up under names like Diethoxydiphenylsilane or silane, diphenyldiethoxy-. Suppliers such as Gelest, Sigma-Aldrich, and others mention these variants along with registry and catalog numbers, making it important to specify exact structure during custom ordering. For those working internationally, translations in major trade languages appear, though silicon, ethoxy, and phenyl roots remain consistent across most systems, minimizing mix-ups.
On the safety front, my own experience reinforces that operators do best by treating every silane as a potential irritant, even when toxicity rates look benign on paper. Ethoxysilanes don’t pack the same punch as chlorosilanes, but operators guard against splashes and inhalation all the same, especially in settings without local exhaust. Spills followed by water can release ethanol and generate slipperiness and local fumes, so spill kits rely on absorbent clay or sand. Waste goes for incineration or specialized chemical waste processing—water treatment plants have no use for hydrolyzed silicon residues. Occupational standards in the US and Europe focus on air monitoring and limiting exposure, with training refreshed yearly to reduce risks linked to handling and pouring.
Diphenyldiethoxysilane serves as a backbone in applications craving durable siloxane structures. In my work consulting for a coatings manufacturer, the compound emerged as a favorite for producing moisture-resistant, high-gloss finishes that withstand years in challenging outdoor environments. Electronics makers treat wafers or glass with this molecule to achieve better adhesion of circuit patterns and improved chemical isolation. Composite materials companies add it as a crosslinking agent to give glass fibers and fillers additional grip within resins, boosting toughness and lifespan of the final part. Its thermal stability opens the door to specialty adhesives and high-performance elastomers—a welcome upgrade for aerospace and advanced automotive design. And research work continues to push boundaries in sol-gel chemistry, making use of diphenyldiethoxysilane’s versatility.
Laboratories investigating new materials pull diphenyldiethoxysilane into their formulations when seeking tunable surface properties and robust silicon-oxygen frameworks. I worked with teams mixing it into hybrid organic-inorganic polymers, watching how it balanced flexibility with abrasion resistance. Researchers continue to chase methods for rapid and selective hydrolysis, aiming to speed up production while minimizing byproducts. In the field of nanotechnology, this silane makes advances possible by anchoring functional groups onto nanoparticles or forming ordered self-assembled monolayers with both hydrophobic microenvironments and long-term stability. Every year, journals document tweaks in reaction conditions, catalyst choices, and application protocols—evidence of a living, breathing R&D ecosystem around this one molecule.
Most toxicity reports and animal studies rate diphenyldiethoxysilane as low-hazard, especially compared to other silicon reagents, but questions still linger about chronic exposure. Acute contact can irritate eyes, skin, and the respiratory tract, a story supported by my own hands-on time in pilot plants. Chronic inhalation hasn’t been shown to cause severe systemic effects, yet modern safety culture insists on minimizing any questionable exposures for workers. Ongoing toxicity reviews track metabolites like alcohols and organosilicon residues. Researchers pay attention to what happens when it breaks down in soil and water—wastewater tests show minimal persistence, but those tests continue as electrification and longer product lifetimes mean more silanes circulate in the environment.
Innovation in advanced materials could push diphenyldiethoxysilane onto bigger stages in the coming years. Next-generation coatings, semiconductor packaging, and flexible electronics look for ever more tailored surface treatments—ones that combine performance with environmental responsibility. My past collaborations with green chemistry teams highlight an ongoing push to optimize production routes: cutting back on solvents, reusing byproducts, and designing processes that operate at lower energy thresholds. As the electronics world spins toward smaller, more complex architectures, companies see value in a molecule balancing thermal resilience, controllable reactivity, and intrinsic UV protection. With additive manufacturing expanding its share of custom part production and sol-gel techniques opening new possibilities for functionalized glass and ceramics, diphenyldiethoxysilane’s future stays closely tied to how creatively scientists and engineers keep reinventing silicon chemistry’s toolbox.
Diphenyldiethoxysilane sounds complicated, but at its core, this compound finds a home in tools and products every day. Silicon-based chemicals often don’t get a lot of attention, but many rely on them. Diphenyldiethoxysilane helps make things tougher, cleaner, or just plain better. I’ve seen it put to work in the world of electronics. It plays a big role as a building block for protective coatings on microchips and circuit boards.
Technology never stands still. As machines and devices shrink, the need for smarter, lighter, and more reliable materials climbs. This is where diphenyldiethoxysilane steps up. It helps form a protective layer through what’s called a silanization process, reducing surface moisture and blocking corrosion.
Have you ever noticed that some plastics last longer or resist cracking under the sun? Diphenyldiethoxysilane often gets mixed into advanced plastics and resins as a crosslinking agent. This means it helps plastics bind together tightly, resulting in sturdier parts—important for things like car components or durable construction materials.
Industrial coatings tap this compound to create a water-repellent or “hydrophobic” surface. Manufacturers use these coatings to shield everything from skyscraper windows to solar panels. Keeping water at bay isn’t just about looks; moisture can cut the life of a product in half. By building up a barrier, diphenyldiethoxysilane keeps surfaces protected much longer.
Chemicals like diphenyldiethoxysilane offer great benefits, but the story doesn’t end with performance. It’s important to consider what happens after a product leaves the factory. Workers handling silanes need training, since the material can irritate skin or eyes, and it’s flammable before it fully reacts in coatings. Facilities often use closed systems and ventilation, because breathing in fumes could cause headaches or more serious effects over time.
For years, researchers and manufacturers have chased safer production and disposal methods for silicon-based chemicals. Companies work to limit releases into the environment by tightening up their processes. More recycling means less waste at the back end. It pushes chemical makers to innovate cleaner paths forward.
People often ask if greener options could get the same job done. Research keeps moving, but not every plant-based or biodegradable chemical can match the performance of silicon-based compounds in tough industrial settings. Colleges and companies keep testing new formulas, though—especially for water repellency and strength. Until they measure up, careful handling and smart design help minimize risk.
Diphenyldiethoxysilane’s role shows how specialty chemicals shape the products we take for granted. By tying together safety, performance, and environmental responsibility, industries keep searching for ways to deliver the best of all worlds. A careful eye on both benefits and drawbacks opens the door to better materials down the road.
ReferencesDiphenyldiethoxysilane sounds intimidating until you break it down. Two phenyl groups, two ethoxy groups, one central silicon atom. As for the actual chemical formula, it reads: C16H20O2Si. You might think of this as two C6H5 (phenyl rings), two C2H5O (ethoxy groups), and a core of silicon. In chemistry classrooms, these formulas set a foundation for understanding structure, reactivity, and risks. I remember sketching similar compounds for hours in organic lab, getting acetone on my jeans. Memorable, but that sort of hands-on work builds trust in the data and formulas we see all over industry literature.
Mistakes in chemical specification don’t end up just on paper. They go straight into labs, factories, and often, products. A solid chemical formula like C16H20O2Si isn’t just trivia; it underpins safe handling guidelines, guides researchers, and helps with regulatory filings. Companies that script their chemical labels with confidence inspire trust in their teams and partners. Scientists facing spills or exposure know what safety glasses or gloves to use based on molecular structure, and first responders want no surprises. That’s real impact—correct formulas support health, safety, environmental stewardship, and innovation.
Diphenyldiethoxysilane usually enters processes that call for organosilicon compounds. In my experience, teams use it for surface modification, adhesion promotion, sometimes in electronics. If someone gets the molecular formula wrong, downstream synthesis can fail. Imagine an engineer in the semiconductor industry relying on precise properties for deposition layers and finding out the weights don’t calculate. Output tanks get filled with wasted material, cash gets burned, and timelines slip.
Researchers also use exact chemical formulas to pull up toxicology studies and reference materials. Getting that formula precise, scientists study the right compound—not something with a typos. Studies on Diphenyldiethoxysilane usually look into hydrolysis, compatibility, and reactivity. If those studies cross wires on formula, safety data sheets end up wrong, leading to missed hazards on the production line. Credible firms track every detail, reviewing nomenclature and results before a molecule even gets ordered for shipment.
Even experienced pros miss steps. Sometimes formulas spread through textbooks or online sources with errors that nobody double-checks. Some databases list compounds only by name without clear formulas. Teaching people to cross-verify everything—formula, molecular weight, CAS numbers—doesn't just apply to grad school. In professional circles, everyone benefits from the discipline of source-checking with primary chemical registries like PubChem or Sigma-Aldrich. Open access to reviewed data smooths out most confusion, but not everyone remembers to use it.
Real fixes help the next chemist or process engineer. Better labeling, stricter data entry protocols, and full electronic traceability make a difference. Peer review within teams, even for something as mundane as a formula, builds confidence for downstream users. Culture shifts toward open correction help, too. Admitting an error in a formula, rather than passing it along, keeps labs, manufacturers, and end-users safe—no egos needed, just curiosity and a bit of humility.
If you’ve ever handled specialty chemicals, you know that storage isn’t a boring afterthought. When working with diphenyldiethoxysilane, safe habits pay off. In the lab, a misstep at the storage stage can turn an easy day into a real mess, not to mention the impact on everyone’s health and budget.
Diphenyldiethoxysilane brings a lot to the table in advanced material science. As a clear, colorless liquid, it often doesn’t look intimidating—but don’t let appearances fool you. This stuff reacts with water, and that gets messy fast. You’re talking about possible buildup of ethanol fumes and the formation of sticky, hazardous siloxane byproducts. That’s not a scenario you want for your workspace or colleagues.
Few things feel as reassuring in a lab as a solid, dedicated chemical storage space. It pays to prioritize areas that keep the chemical cool, dry, and away from sunlight. Heat speeds up unwanted decomposition and even boosts the pressure inside a container. A shady, well-ventilated shelf or cabinet, far away from steam lines or radiators, fits the bill.
Moisture spells trouble for diphenyldiethoxysilane. Even low humidity can set off slow hydrolysis, cutting the shelf life and risking dangerous gas release. I always go for airtight containers—preferably glass or compatible plastics with top-notch seals. As an extra step, throwing in some desiccant helps soak up any stray humidity. Using this approach, I once kept a shipment in usable shape for over a year, even during a tough, muggy summer season.
Labels sound boring, but mistakes here create headaches later. I’ve seen expensive batches ruined when a tech stored different silanes together and mixed up the labels. Everything should have the full chemical name, date received, and important hazard symbols right up front. Putting diphenyldiethoxysilane next to strong acids or bases is asking for trouble, too. Keep incompatible substances separated. Better to walk an extra few steps than reach for the emergency kit.
Strong fumes never helped anyone’s focus. Working with airtight containers in a ventilated storage cabinet cuts down the risk of inhaling vapors. Make sure the cabinet exhaust points outside, not back into the workspace. It’s one simple step for a safer environment, especially if you deal with these chemicals day in and day out.
Storing diphenyldiethoxysilane in the perfect environment counts for little if you forget about it for years. A monthly inspection works wonders for catching broken seals, cloudy liquids, or pressure buildup. If something seems even a little off, don’t keep it in storage. Safe disposal beats a sudden leak or blown container every time.
Reliable storage doesn’t just tick boxes—it saves time, money, and maybe even lives. Every smart step—tight lids, clear labels, dry storage, proper shelving—builds up trust and safety in your lab or warehouse. It’s about protecting both products and the people who handle them. In my years working with specialty chemicals, careful handling always paid off, giving peace of mind to everyone involved.
Diphenyldiethoxysilane can do a lot of good in the right setting, but if you overlook basic safety, trouble follows. Working with this chemical in the lab lets me see firsthand how easily things can go sideways. This is a clear, often slightly yellowish liquid that releases fumes—fumes that sting the nose and eyes before you even notice. People may think gloves and glasses offer enough. They don’t.
This compound can irritate the skin and eyes right away. You inhale the vapor, your lungs pay. Let it linger on your hands and the burn tells its own story. Once, in college, I saw a friend splash a little on his arm. He brushed it off. Within seconds, he ran for the sink. Ever since, I double-check gloves and sleeves before uncapping the bottle. Burns are hard to forget. I found out most accidents happen because someone skips a step. Even trained chemists rush, thinking it’s just one more routine transfer.
Wear splash-resistant goggles, not just basic safety glasses. I wore glasses for a year before learning the hard way—liquids find the gaps around regular lenses. I always put on a lab coat with fitted cuffs and pair it with nitrile gloves. If there’s even a chance of a spill, I grab a face shield and make sure my pants cover my ankles.
Work near an eyewash station. I once had to lead a coworker to the station with his eyes sealed shut from a splash. You remember moments like that. Check that the area has strong airflow. Fume hoods change everything. I once tried a fume hood with the sash cracked too high. Fumes drifted out, set off the alarms, and we all cleared out for twenty minutes. Keep the sash at the right height. If there’s any odd smell, clear out until you know what’s going on.
Store the bottle in a cool, dry place. This chemical reacts with water in the air. I saw glassware cloud up after someone left a bottle open during a humid day. We spent hours scrubbing the residue. Good containers matter. Tightly seal the cap after every use, and label everything. So many times, I’ve opened a random container, sniffed, and regretted it. Labels protect the whole lab, not just the person who poured the chemical last.
Plan what to do if things go wrong. Spills don’t announce themselves. I keep a chemical spill kit under my bench, not across the room. Use absorbent pads and scoop up any residue right away. Don’t ever touch liquid spills directly. Shovel the materials into a waste container and keep the lid tight. My old supervisor always said, “Treat every spill like an emergency, even if it looks tiny.”
Disposing of diphenyldiethoxysilane means following hazardous waste laws. Never pour it down the drain. Each lab has a protocol, but the wrong move can lead to hefty fines or, worse, sewer accidents. If you’re unsure, ask the safety officer. I’ve seen smart people lose their tempers arguing with disposal teams—best to read the rules and skip the drama.
Safety in the lab isn’t solo work. I always mention risks to new teammates, even if they think I’m overcautious. Trust comes from shared experience and a bit of stubborn repetition. Every day without an accident is a win for everyone in the room. If you’re careful with diphenyldiethoxysilane, you protect yourself and the whole team. That’s reason enough to take the extra time.
Diphenyldiethoxysilane doesn’t ring serious alarm bells for most people, but that doesn’t mean it can be handled without care. It’s part of the large family of organosilicon compounds, which pop up in industries from electronics to specialty coatings. Most people won’t encounter it in daily life, but folks working in chemical labs or materials manufacturing probably know it well. The safety data available today mostly comes from research settings and workplace safety references, since everyday products rarely use this chemical in their finished form.
Handling this compound isn’t like spilling a bit of vinegar on your skin. If you touch it directly, you could get some irritation, redness, or discomfort on the skin and in your eyes. Safety sheets rate it as an irritant. The vapor may bother your lungs and throat if you’re working in close quarters without good ventilation. Anyone spraying or heating it without protective gear risks coughing, shortness of breath, or headaches—symptoms common to many industrial solvents and silanes.
Long-term studies on human exposure haven’t been published, so there aren’t many details about bigger health problems like cancer, genetic issues, or hormone effects. With my years keeping up with industry safety procedures and following the OSHA framework, I’ve noticed most chemical producers treat silanes like diphenyldiethoxysilane with extra respect: goggles, gloves, chemical-resistant aprons, and ventilation fans all stay close at hand. That routine tells me enough—we may not know every risk yet, but getting careless isn’t an option.
Most chemical spills cause lasting damage when they seep into soil or water. Diphenyldiethoxysilane, on its own, does not usually hang around in the environment for years like PCBs or PFAS, but that doesn’t make it harmless. In contact with wet air, the substance breaks down into phenol, ethanol, and silicon-containing compounds. Phenol and ethanol can run into rivers and streams, disrupting aquatic life. Fish and amphibians absorb these run-off chemicals easily, which messes with their systems. No one wants to see a lab leak take out a local minnow population, and ignoring byproducts like phenol is a mistake.
Soil bacteria help clean up some of the residue, but not every ecosystem recovers at the same speed. Studies show that silanes, over time, often turn into forms that stick to minerals in dirt or sand, so they don’t stay floating in the water forever. That cuts down on how much wildlife gets exposed, but even small amounts can tip a delicate balance.
Better safety starts on the factory floor and in the shipping process. Caps stay tight, containers remain upright, and team members know exactly what to do in case of a spill. I’ve seen quality labs invest in real-time air testing, not just for worker comfort but to catch leaks before fumes fill the room. Waste collection keeps chemical run-off from heading down the drain, and any big batch gets sent out to certified disposal teams instead of trusting the local landfill.
Transparency matters here. Regular training for everyone, not just chemists, helps prevent accidents. Engineers and managers keep up with changes in chemical laws, so if the EPA or the European Chemicals Agency updates the hazard profile, they don’t miss a beat. Pushing companies to switch to less risky chemicals or more eco-friendly versions when possible goes a long way. Pressure from buyers, workers, and the public keeps chemical safety off the back burner.
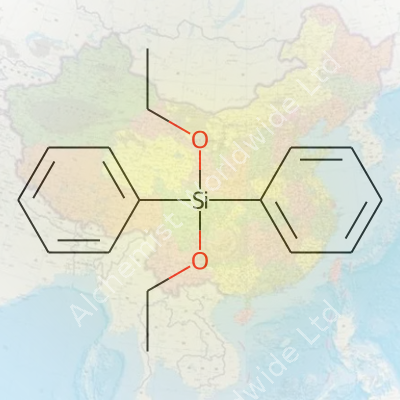
| Names | |
| Preferred IUPAC name | diethoxy(diphenyl)silane |
| Other names |
Diethoxydiphenylsilane Diphenyldiethoxy-silane |
| Pronunciation | /daɪˌfiːniˌlˌdaɪˌɛθɒksiˈsaɪleɪn/ |
| Identifiers | |
| CAS Number | [2555-14-6] |
| Beilstein Reference | 1460081 |
| ChEBI | CHEBI:87356 |
| ChEMBL | CHEMBL4298977 |
| ChemSpider | 128857 |
| DrugBank | DB22435 |
| ECHA InfoCard | 03-2119957104-51-0000 |
| EC Number | 4119-60-4 |
| Gmelin Reference | 83294 |
| KEGG | C19343 |
| MeSH | D009074 |
| PubChem CID | 131740 |
| RTECS number | VV5950000 |
| UNII | 75D9F46A16 |
| UN number | UN2312 |
| Properties | |
| Chemical formula | C16H20O2Si |
| Molar mass | 302.43 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 1.045 g/mL at 25 °C(lit.) |
| Solubility in water | Insoluble |
| log P | 3.9 |
| Vapor pressure | 0.2 hPa (20 °C) |
| Acidity (pKa) | 20.9 |
| Basicity (pKb) | 3.98 |
| Magnetic susceptibility (χ) | -69.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.504 |
| Viscosity | 1.7 mm2/s (25°C) |
| Dipole moment | 2.20 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 383.9 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P301+P312, P305+P351+P338, P337+P313 |
| Flash point | 110°C |
| Lethal dose or concentration | LD50 (oral, rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 2200 mg/kg |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Diphenyldiethoxysilane: "Not established |
| REL (Recommended) | 3 ppm |
| Related compounds | |
| Related compounds |
Diphenyldichlorosilane Triphenylsilane Diphenylmethylsilane Diphenylsilane Phenyltriethoxysilane |