
Chemists searching for versatile building blocks often discovered organosilicon compounds by accident in the busy labs of the early 20th century. Diphenyldimethoxysilane has roots in efforts to bridge organic and inorganic chemistry by adding silane groups to aromatic structures. As interest grew around the 1950s in silicone materials, researchers singled out this compound for its adaptability. Its rise didn't simply come from theory. There was a real hunger for substances that could handle the demanding roles in both industrial and lab settings. As experienced colleagues have told me, work on derivatives like this often sprang from simple curiosity and the realization that a molecule with both phenyl and siloxy features carried appeal for those trying to push beyond well-trodden carbon chemistry.
Diphenyldimethoxysilane shows up as a clear, colorless liquid with a sharp, unmistakable chemical odor. At first glance, it doesn’t look much different from many silane reagents. The power hides in its simple formula C14H16O2Si, with two methoxy groups and a pair of phenyls attached directly to silicon. Producers often label it as a moisture-sensitive product, since it tends to break down in the presence of water. Handling always takes a careful hand and airtight equipment, something students in synthetic labs quickly learn about when a flask fogs up too early. Various chemical suppliers offer it under names like dimethoxydiphenylsilane and silane, diphenyldimethoxy-, listing it with a CAS number for traceability.
Diphenyldimethoxysilane shows a boiling point close to 148-150 °C at atmospheric pressure and a density around 1.07 g/cm³ at 25 °C. Its refractive index hovers near 1.52, giving it a certain sparkle under a bench lamp. The molecule dissolves easily in many organic solvents but repels water, and the methoxy groups react quickly with moisture or acids. It's not the sort of substance that lingers on a bench overnight, especially during humid weather. As I recall from my own research, the smell can linger longer than you’d think if somebody leaves the bottle open. Chemical reactivity comes mostly from the silicon-oxygen bonds, which break and form just as readily as any eager chemist would hope.
Bottles supplied by manufacturers bear hazard pictograms highlighting both flammability and chemical reactivity. Batch certificates spell out purity, which usually exceeds 98% for most synthetic work. Labels warn plainly about the risks of contact with eyes, skin, and the respiratory tract. Documentation keeps clear about storage needs—tightly closed, inert atmospheres, and away from strong oxidizers or acids. Just a few milliliters, if handled carelessly, can attack metal or glassware through hydrolysis. This reinforces the importance of trained handling, much like dozens of other volatile organosilicon agents I’ve seen stored behind locked cabinets.
Synthesis commonly begins with diphenyldichlorosilane, where methanol treatment in the presence of a base yields the dimethoxy derivative. It's a textbook example of nucleophilic substitution: chloride gives way to methoxy, often under the cooling hum of an ice bath, as hydrogen chloride gas escapes. The product separates cleanly with non-aqueous extraction. Inefficiencies creep in with water contamination, so researchers take extra steps to dry everything thoroughly, using desiccators or molecular sieves to dodge yield losses. The thrill comes at workup, where a clear layer settles in, showing off the success of such a simple yet satisfying transformation.
The story of this molecule continues in how easily it trades its methoxy groups for other nucleophiles—a trait that makes it essential in surface modification chemistry. On glass or metal oxide surfaces, it anchors phenyl groups with stability. It reacts cleanly with alcohols, acids, or even mild water, forming silanols first and eventually more robust siloxane linkages. Teams designing organic-inorganic hybrid materials use this to engineer layers and interphases you just don't find anywhere else. I remember colleagues functionalizing magnetic nanoparticles using this very type of silane—watching the IR spectra shift as new bonds formed, signaling success right away.
Across catalogs and regulatory documents, diphenyldimethoxysilane carries many alternate labels: 1,1-diphenyl-1,3-dimethoxysilane, diphenylsilicon dimethoxide, or simply DPDMS. Each identifies the same chemical backbone, which remains recognizable by both casual chemists and regulatory inspectors. The importance of accurate naming stands out most during international shipping—a missed alias can hold up a project for weeks, as I once learned the hard way dealing with import paperwork for a collaboration overseas.
Working with diphenyldimethoxysilane calls for full personal protective equipment: gloves, goggles, and lab coats. Fume hoods prevent inhalation of its vapors, as exposure can irritate the eyes and lungs or cause burns. Facilities train staff in safe storage, spill response, and first aid, emphasizing the need to keep acids, bases, and oxidizers clear of open bottles. Eye-wash stations and chemical spill kits stay close at hand. I’ve seen firsthand how urgent a response becomes when even a small amount spills on skin—it stings immediately and motivates thorough training and proper respect for all chemicals, no matter how seemingly routine.
Engineers and scientists rely on this silane for surface treatments that improve adhesion between organic resins and mineral fillers. In microelectronics, it acts as a coupling agent, prepping silicon wafers for further assembly. Polymer chemists appreciate its use in cross-linking, which boosts mechanical properties and longevity in specialty plastics or rubber. I’ve seen coatings that resist heat and moisture perform especially well after surface modification using organosilicon molecules just like this one. Its reach includes thin film fabrication, high-performance composites, and even specialty glassware manufacturing. Researchers working on nanotechnology favor its reliable and predictable surface reactions, letting them fine-tune particle properties for targeted medical or environmental applications.
Academic labs and industrial innovation centers chase new modifications of diphenyldimethoxysilane. Teams tweak its structure to change solubility, reactivity, or compatibility with new polymers. Materials scientists investigate how varying the aryl or alkoxy groups can yield more resilient composites for automotive or aerospace use. Method development doesn’t stop, as advances in catalysis or green chemistry push for more efficient and safer approaches. Cross-institutional collaborations keep surfacing, with partners sharing insights gained from everything from analytical techniques to large-scale synthesis. Every batch, each experimental result, folds into the next round of research, turning what seems like a mature compound into a springboard for discovery.
Toxicologists have tracked the effects of diphenyldimethoxysilane on both human and environmental health. Acute exposure can irritate the eyes, nose, and throat, especially if vapors escape containment. Chronic data remains sparse, though animal studies suggest that repeated skin contact should be avoided due to potential for sensitization. Environmental breakdown leads to phenyl and methoxy byproducts—scientists have measured these in accidental spill scenarios, watching for longer-term aquatic impacts. Regulatory agencies require careful documentation, reflecting the community’s consensus that prevention takes priority. In real lab settings, the best practice stays focused on double-checking procedures before each use, minimizing unnecessary risk.
Materials science keeps driving demand for silane compounds that do more than bridge gaps. The next decade will bring more sustainable production routes, thanks to biodegradable catalysts and renewable carbon sources feeding into silane synthesis. Downstream, tailored surface modifications—enabled by diphenyldimethoxysilane derivatives—promise adhesives that perform without relying on toxic additives. Electronics industries want to tune interfaces at the molecular level, unlocking better performance and fewer failures. Environmental regulations will tighten, so industry leaders prepare for greener, lower-toxicity alternatives and responsible waste management practices. Chemical innovation, as history keeps showing, won’t slow down, and builders of new molecules stand to find fresh purpose for what might look like an old compound.
Diphenyldimethoxysilane doesn’t exactly roll off the tongue. Yet this clear, colorless liquid quietly shapes a surprising number of things people use. Its main job lies in the world of organic synthesis, especially as a building block in the creation of specialty siloxane polymers and resins. I’ve spent years around manufacturing floors and research labs, and I’ve seen firsthand how this compound brings flexibility and toughness to silicones, making coatings last longer and electronics more reliable.
The big draw with diphenyldimethoxysilane comes from its silane group and two phenyl rings. That chemistry gives industries a way to add strength and toughness to finishes without giving up transparency or flexibility. In paints and coatings, this means protective layers don’t crack or turn yellow with sun exposure. Manufacturers of waterproof electronics depend on these qualities to keep moisture out of precision circuitry.
Glass fiber and plastic manufacturers also look for this compound when treating surfaces. It helps glue resin to glass fibers, producing composites that hold up in airplanes or cars at highway speeds. I’ve spoken to engineers who lean on these treatments because they trust them to prevent failure from creeping in over time.
People often ask me if they’ve come in contact with chemicals like this in daily life. They have, even if they didn’t know it. This compound often finds its way into silicone sealants. Think about aquariums, window caulks, or the flexible phone cases so many people carry. It stands out for not reacting with water too quickly, which makes it easy to control during the manufacturing process.
Adhesives and insulating foams also depend on diphenyldimethoxysilane. Construction workers use these materials for energy-efficient buildings, and the firefighters I’ve met appreciate safety gear that uses heat-resistant silicone coatings.
Any chemical with broad reach brings responsibility. This is where my own experience rings true: I’ve seen environmental health and worker safety policies tighten over the past decade. Reliable sourcing, good ventilation, and training keep accidents rare — but ongoing investment matters. The American Chemistry Council and health agencies continually review data on chemicals like diphenyldimethoxysilane, guiding companies toward lower risk and better transparency.
For companies that use this compound, the right move is to put clear, practical safety steps in place on the shop floor. Storage containers need labeling, spills get managed with training, and disposal follows local rules. Community awareness and open reporting shut down the “black box” problem, where only insiders know what’s really happening. Many firms have started publishing sustainability reports, giving people honest information about the chemicals in use and what’s being done to keep air and water safe.
Research into cleaner production of silanes goes on. Green chemistry teams push for routes that cut out harsh byproducts or lower energy use, helping align industry goals with environmental needs. Sometimes new catalysts or smarter designs trim waste right at the source. I’ve toured next-generation labs where students try to invent ways to reclaim and reuse byproducts that, in the past, might have been discarded. Engineers, managers, and policymakers have the experience and tools to move things in a better direction without giving up performance or reliability in everyday products.
Chemistry isn’t just something locked away in a lab; it shapes all kinds of industries and even things you find in ordinary life. Diphenyldimethoxysilane has made its mark as a key building block in specialty silicones and advanced manufacturing. Its formula—C16H18O2Si—shows up in research papers, synthesis guides, and product specifications, but the story gets more interesting once you start looking beyond just the letters and numbers.
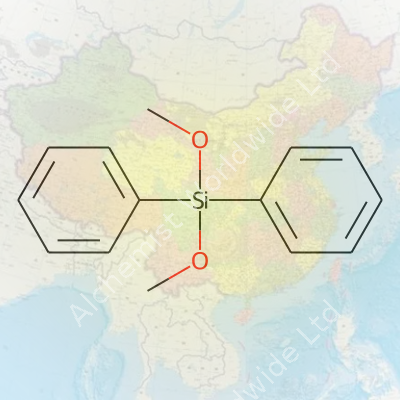
Silanes with different organic groups shape how coatings stick, how electronics keep moisture out, and even how certain plastics get their shine. In my experience working with specialty chemicals, materials like diphenyldimethoxysilane let chemists tweak reactions just the way they want. The structure brings together two phenyl groups and two methoxy groups attached to a central silicon atom. So, that chemical shorthand isn’t just a formula—it gives a window into how the molecule reacts, what it can bond with, and why manufacturers care.
The structure—two C6H5 rings joined to silicon by direct bonds, plus two OCH3 groups on silicon—makes this compound a versatile player. With the methoxy parts, it’s possible to build larger molecules; the phenyl rings add rigidity and resistance to weathering, heat, and certain chemicals. I’ve watched teams use this know-how to design better water-repellant coatings, stronger adhesives, and even certain polymers used in niche electronics.
Raw formulas only take you so far. Handling chemicals like diphenyldimethoxysilane demands understanding what happens during storage, mixing, spills, or fires. Methoxy groups easily react with moisture; this can release methanol—a toxic, flammable byproduct. From working in research settings, I’ve learned that knowing the chemical structure not only speeds up new product development but keeps everyone safe. Research groups and safety agencies point to the risks of methanol exposure—eye and respiratory irritation, even serious health effects at higher doses.
Manufacturers want materials that last longer, perform more reliably, and hold up to use in tough environments. Silanes with phenyl and methoxy groups stand out in this regard. For example, electronics manufacturers often look for ways to keep water and dust out of sensitive parts. Diphenyldimethoxysilane becomes valuable here since its unique structure lets it form tough protective films. I’ve seen firsthand how controlled use of this compound can shrink product failures and extend useful life. There’s a ripple effect—fewer replacements, less waste, and better trust in finished goods.
Smart handling means staff training, up-to-date safety data sheets, proper personal protective gear, and active monitoring for leaks and spills. Companies and regulatory groups constantly review best practices. I’ve noticed more facilities investing in automation to reduce direct worker contact, as well as updated ventilation and containment systems. Many teams also test substitutes or blend silanes to get the right balance of safety and performance. Open communication—sharing near-misses, updating procedures, talking openly about mistakes—goes a long way in building reliable, safe innovation.
Behind every chemical formula stands a set of choices that ripple through performance, safety, manufacturing, and even sustainability. Diphenyldimethoxysilane—C16H18O2Si—offers a vivid example of how chemistry, experience, and careful planning shape the products and technologies we rely on every day.
Diphenyldimethoxysilane shows up in labs and industrial spaces more often than people might guess. This compound has silicon in a form that attracts both innovation and risk. Over the years, working with chemicals like this, I’ve seen both safe storage and sloppy mistakes. The risks usually don’t announce themselves with a bang; they sneak up later—think leaks, yellowing, or that sharp smell nobody wants near their face.
The stuff thrives in cool, dry places. Stash it somewhere people can count on for steady temperatures, below 25°C, but definitely above freezing. Humidity spells trouble. Any extra water in the air can start a slow reaction, releasing methanol gas. Even small leaks from a loose cap can lead to dangerous fumes in a closed room. I remember a colleague who shrugged off the warning and set a couple bottles on the windowsill above a radiator. By the end of the week, the room reeked, and half the stock looked cloudy.
A tight lid makes a world of difference. Use original packaging if it came sealed right, or rely on high-density polyethylene, glass, or Teflon-lined containers. No one wants to discover a warped or crumbling plastic jug after a weekend away. I learned quickly—never store this stuff in metal. Methanol can corrode through most lids and start a slow disaster.
Fresh air isn’t a luxury for Diphenyldimethoxysilane. A well-ventilated storage area avoids vapor buildup. Fume hoods and local exhaust systems do more than make the job easier—they prevent mishaps you can’t undo. Most lab accidents I’ve seen started in cramped rooms with little air flow and bad storage habits.
Even though Diphenyldimethoxysilane doesn’t flame up just sitting there, its fumes and breakdown products catch fire easily. Keep it away from open flames, hot plates, and even electrical outlets with loose wiring. Fire-resistant storage cabinets, spaced clear of ignition sources, limit risk. I always kept a fire blanket nearby, and once it stopped a small spill from turning into a catastrophe.
Diphenyldimethoxysilane doesn’t play nice with acids, oxidizers, or anything wet. Store it alone or with other organosilanes only. One time, a shelf got reorganized, and a bottle ended up next to a leaking acid. The cloud that formed made us evacuate the lab and lose days rechecking all the chemicals for cross-contamination.
Losing track of what’s on a shelf invites accidents. Every bottle deserves a clear label—contents, hazard symbols, the date it arrived. Dated inventory logs keep old material from lingering. I’ve seen expired or forgotten chemicals become ticking time bombs after sitting too long. A tight inventory stops that problem at the source.
Diphenyldimethoxysilane’s hazards make it a substance for trained hands only. Restricting access works better than signs or warnings. I used to hold short refreshers for new staff, emphasizing the quirks of silanes and the real risks of old habits. The right knowledge and respect do more to prevent incidents than any rulebook alone.
Standing in a lab or working on a factory floor, you run into chemicals with names that hardly roll off the tongue. Diphenyldimethoxysilane fits right in—used in industrial coatings, silicone polymers, or as a building block in science labs. You unpack a bottle, glance at the label, and notice warnings. Now, your common sense and safety training kick in, so let’s talk about whether this chemical deserves that caution.
In the lab, opening containers of organic silanes reminds me of my early days—smelling that distinctive solvent hit, seeing the way a drop spreads on a glove. Most folks don’t realize that Diphenyldimethoxysilane, like many silanes, doesn’t play nice with water or skin. It hydrolyzes, forming methanol and releasing heat. Methanol vapors irritate eyes and lungs; skin contact feels like a mild burn or tells your nose you've made a mistake. Handling this stuff unsafely may give chemical burns, headaches, or worse if inhaled over time due to methanol exposure.
The manufacture and research involving Diphenyldimethoxysilane often means small spills and splashes. I’ve seen how a moment’s inattention—like skipping goggles or using thin gloves—leads to red, stinging skin, or worse, a trip to the emergency room. People get overconfident, thinking nothing bad happens in ‘ordinary use.’ But one slip with Diphenyldimethoxysilane, mixed with sweat or humidity, can change that.
Reviewing safety data sheets, I always notice clear mentions of “flammable,” “causes burns,” and “forms toxic fumes.” The National Institute for Occupational Safety and Health and Occupational Safety and Health Administration set tight limits for many silanes for good reasons. The methanol produced attacks your nervous system, while phenyldimethoxysilanes often irritate skin and eyes. The risk isn’t as dramatic as some industrial acids or cyanides, but if safety controls slip—poor ventilation, lack of gloves, or leaving spills unchecked—the results pile up over time. Chronic exposure isn’t visible like a flash fire, yet the toll adds up in headaches, rashes, or even nerve damage.
In practice, the answer isn’t about avoiding Diphenyldimethoxysilane altogether. The trick lies in taking the warnings seriously. Chemical fume hoods, goggles, and gloves rated for organic solvents make the difference here. Companies with decent training make sure anyone handling silanes respects the threat—no casual shortcuts, no open bottles on the bench near liquids or food. Clear air exchange systems cut back methanol vapor. I’ve seen small teams run safe and smooth with basic gear and some discipline, while cost-cutting leads to messy records and more “incidents.”
Training everyone on what to do during spills or accidental exposure helps, not just reading the policy off the wall. Keeping emergency wash stations close by and using disposable pipettes or syringes keeps accidents rare. Good supervisors set expectations and make sure people respect both the risks and themselves by using the right gear every time.Diphenyldimethoxysilane isn’t the most dangerous chemical on the shelf, but it commands respect. Treating it casually only works until it doesn’t. The right protocols and gear, sturdy routines, and a bit of humility keep you healthy and out of urgent care. Over decades in labs and plants, that adds up to far fewer stories about near-misses and a lot more about clean, safe workdays.
Talking about diphenyldimethoxysilane, folks in research and manufacturing don’t just ask what it is—they dig straight into purity. Working in a lab, I've witnessed the difference a single percentage point in purity can make. Imagine pouring weeks of work into an experiment, only to have odd side products ruining the result. With silanes, that risk multiplies: contamination means poor adhesion, unpredictable reactions, or even complete failure of coatings or electronics.
Purity, in this context, boils down to how much of the material in the bottle is exactly diphenyldimethoxysilane and not something else. Most chemical suppliers aim for assay values over 98%. For high-end applications like advanced materials research, semiconductor work, or pharmaceutical intermediates, purity often climbs to 99% or higher. Anything less, and you're rolling the dice on side reactions or poor yields.
A typical bottle or drum will carry a certificate of analysis. Here’s what’s usually listed:
I’ve worked on coatings where one batch of low-purity silane left a factory floor sticky for weeks. In electronics, poor purity means inconsistent dielectrics and device failures. Even in relatively forgiving fields—like paint and plastics—bad silane wastes time and money by gumming up processes or dropping yields. Researchers regularly fork over extra funds for ultra-pure silanes, just to dodge headaches later.
Every reputable source offers detailed certificates and independent lab results, not just marketing promises. Labs verify these numbers with NMR, GC-MS, and Karl Fischer titration. Once bottles arrive, storage makes an equally big difference. Air and moisture sneak in fast, so folks use dry boxes, argon blankets, and sealed containers. I’ve lost reagents before when they turned milky, just because somebody left a cap loose overnight.
Suppliers can tighten their process by investing in better distillation and quality control. End users can demand certificates with every lot. Sharing feedback on inconsistencies helps push the industry forward. Labs can test a small sample themselves before scaling up. Just a few simple steps save time, money, and frustration down the road.
| Names | |
| Preferred IUPAC name | diphenyl(dimethoxy)silane |
| Other names |
Dimethoxydiphenylsilane DPMOS Diphenylsilicon dimethoxide |
| Pronunciation | /daɪˌfɛnɪlˌdaɪˌmɛθɒksiˌsaɪleɪn/ |
| Identifiers | |
| CAS Number | 6843-66-9 |
| Beilstein Reference | 136187 |
| ChEBI | CHEBI:87135 |
| ChEMBL | CHEMBL2281372 |
| ChemSpider | 81805 |
| DrugBank | DB14657 |
| ECHA InfoCard | 100.083.548 |
| EC Number | 211-189-2 |
| Gmelin Reference | 2090221 |
| KEGG | C19229 |
| MeSH | Diophenyldimethoxysilane |
| PubChem CID | 68539 |
| RTECS number | VV4950000 |
| UNII | 00W8T41LY2 |
| UN number | UN2668 |
| CompTox Dashboard (EPA) | DTXSID6047342 |
| Properties | |
| Chemical formula | C14H16O2Si |
| Molar mass | 242.36 g/mol |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 1.06 g/mL at 25 °C (lit.) |
| Solubility in water | Insoluble |
| log P | 2.9 |
| Vapor pressure | 0.008 mmHg (25 °C) |
| Acidity (pKa) | Acidity (pKa): 35.4 |
| Magnetic susceptibility (χ) | -68.0e-6 cm³/mol |
| Refractive index (nD) | 1.546 |
| Viscosity | 0.84 cP (25°C) |
| Dipole moment | 2.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 304.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -7020 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P301+P312, P304+P340, P312, P403+P233 |
| NFPA 704 (fire diamond) | 1-2-1-ℙ |
| Flash point | 85°C |
| Autoignition temperature | 530 °C |
| Lethal dose or concentration | LD50 (Oral, Rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 2200 mg/kg |
| NIOSH | GV5950000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | No REL established |
| Related compounds | |
| Related compounds |
Diphenylsilane Tetraphenylsilane Dimethyldimethoxysilane Diethyldimethoxysilane Phenyltrimethoxysilane Phenyltriethoxysilane |