
Organic chemists in the twentieth century started relying on new silylation agents, and among these, hexamethyldisilazane (HMDS) rapidly gained traction. Demand for more robust, water-resistant, and surface-modifying chemicals took off in the mid-20th century, especially with the expansion of semiconductor technology. The push for more advanced analytical tools during the 1960s and 70s drew attention to compounds like HMDS, as researchers sought reagents able to protect sensitive functional groups and treat surfaces that modern protocols demanded. Production and use scaled rapidly as industries realized the advantages HMDS brought to both laboratory and industrial workflows.
Hexamethyldisilazane, often shortened to HMDS, stands out as a colorless to slightly yellowish liquid with a persistent ammonia-like odor. In my experience working with analytics labs, small bottles of HMDS often stay close at hand for prepping glassware and making samples hydrophobic before trace analysis. In electronics manufacturing, process engineers count on it for its ability to modify silicon wafer surfaces with a thin, durable film that increases photoresist adhesion, critical for the sharp lines and reliable layers on modern chips. Suppliers list it under many names, but wherever it’s used, its silazane structure makes it valuable for transforming and protecting sensitive functional sites.
Hexamethyldisilazane appears as a clear, nearly transparent liquid, known for its low boiling point—just over 125°C. The density hovers around 0.77 grams per cubic centimeter, and the compound shows limited solubility in water; on contact with moisture, it reacts quickly and liberates ammonia, turning hydrophilic surfaces into hydrophobic ones within seconds. Flammable vapors pose risks around open flames or heat sources; with a flash point near 17°C, labs using it often work with strong ventilation and strict storage protocols. Despite its low viscosity and high volatility, HMDS offers surprising chemical stability in anhydrous conditions, making it durable for storage if well-sealed.
Commercial HMDS typically comes meeting assay standards above 98%. Common labeling requirements include hazard pictograms for flammability, environmental hazards, and acute toxicity, since it can irritate the respiratory system and skin. Barcode systems and QR code traceability have replaced much of the old permanent marker labeling, largely to monitor inventory due to its regulated status in some regions. Standard packaging ranges from 100 mL amber-glass bottles for laboratory use up to industrial drums in chemical manufacturing settings. Product lots carry batch numbers, manufacturing dates, and safety certification information—key details for tracking performance variations in critical production environments.
Commercial routes to HMDS usually follow the methylation of chlorosilanes. In most cases, mixing ammonia with trimethylchlorosilane causes the ammonium chloride byproduct to form, which can be filtered off, leaving behind relatively pure HMDS. Synthetic chemists often appreciate the reliability of this pathway: it lends itself to scale, and waste control technologies capture and neutralize evolving ammonia, keeping environmental impact modest. Precision in temperature and reactant purity has a noticeable effect on yield and product quality, meaning that larger-scale plants install process controls and flow reactors to limit unwanted side reactions.
Hexamethyldisilazane reacts energetically with water to produce ammonia and silanols, making it useful in protocols that need in situ ammonia generation or controlled hydrophobization. In organic synthesis, HMDS works as a silylation agent for alcohols, amines, and acids, converting them into their trimethylsilyl (TMS) derivatives—compounds often used in gas chromatography because they show less tailing and better volatility than their unprotected forms. The reaction with acids produces trimethylsilyl esters, and with alkynes or organometallics, HMDS can act as a base or a ligand source, broadening its versatility in more advanced synthetic chemistry. Process chemists have pushed the envelope by crafting modified silazane frameworks from HMDS, targeting specialty coatings and unique gas-phase precursors for chemical vapor deposition.
In catalogs, Hexamethyldisilazane goes by many names: 1,1,1,3,3,3-Hexamethyldisilazane, HMDS, and bis(trimethylsilyl)amine. Industry colleagues might refer to it by abbreviated codes, even “Silyl Amine,” depending on the context. This variety often leads to confusion in ordering or storage, especially in facilities that handle multiple silazane reagents side by side. International regulatory lists assign it numbers like CAS 999-97-3, helping standardize specifications for global commerce. Producers often use proprietary branding on bulk products, reflecting competing grade standards tailored for electronics, chromatography, or coating markets.
Workplaces handling HMDS take fire risk seriously. I’ve seen entire procedures rewritten after a near-miss with misted vapors around a spark. Storage protocols rely on flammable liquid cabinets, away from acids and sources of ignition, and clear labeling with hazard pictograms stays updated as regulations shift. Spill control procedures call for absorbent pads and full-face respirators to avoid respiratory irritation from ammonia, which releases instantly if even a small amount of water contacts the liquid. Engineering controls—like closed transfer systems and nitrogen blanketing—get used in high-volume operations, reducing worker contact and minimizing atmospheric emissions. Waste gets segregated because residues can foul up municipal treatment plants or spark regulator interest.
Hexamethyldisilazane has become essential in microelectronics, where it primes silicon wafers for photoresist application in photolithography, improving device yields and reducing process drift. In analytical labs, trace chemists treat glassware and microscope slides with HMDS to keep moisture interference and sample adsorption down to a minimum, gaining quality data from GC and GC-MS runs. Some research groups in polymer science depend on it for surface treatments, creating non-stick coatings and modifying nanoparticles for biomedical work. It’s also vital for producing TMS-protected derivatives, often an enabling step in total synthesis campaigns. Environmental scientists, on the other hand, sometimes frown on HMDS because its reactivity poses runoff and airborne risks if not contained.
Academic groups have published a steady stream of papers on new uses for HMDS, focusing on surface passivation, inert coatings, and advanced organosilicon frameworks with custom-tailored functions. Ongoing studies in electronic device fabrication examine whether other silazanes can outperform HMDS for extreme ultraviolet (EUV) lithography, but so far, the compound remains hard to beat for its straightforward chemistry and reliable film formation. Collaborations between universities and industry tweak the base HMDS structure, searching for derivates with lower toxicity, higher selectivity in silylation, or lower vapor emissions. Funding agencies increasingly ask about environmental impact, pushing chemists to revisit waste streams and lifecycle analysis for this once-overlooked reagent.
Scientists have devoted significant effort to understanding the inhalation, dermal, and environmental toxicity of HMDS. Acute exposure studies in rodents show respiratory and neurological irritation at high doses, correlating with symptoms seen in unprotected workers accidentally exposed during spills. Chronic studies find little evidence for bioaccumulation, but environmental reactivity still raises questions about the byproducts made when HMDS enters waterways or soil. Wastewater treatment plants sometimes struggle to break down HMDS fully, creating regulatory concerns for sites near sensitive ecosystems. Manufacturers responded by issuing robust safety instructions and increasing the availability of safety data, aligning with shifting international standards about chemical management and transparency.
The path ahead for Hexamethyldisilazane seems closely tied to the evolution of high-tech manufacturing and analytical science. Growing demand for miniaturized and more powerful semiconductor chips almost guarantees that HMDS—in its current or an improved form—remains central in surface preparation. Researchers look for greener synthesis methods, either improving ammonia capture or deploying alternative silylation agents that lower the environmental footprint without sacrificing performance. High-throughput screening of HMDS analogs could reveal new materials suited for biocompatible coatings, battery technologies, or even as feedstocks for flexible electronics. Regulators and industry safety boards keep tabs on exposure limits and push companies toward even tighter controls, ensuring that as its uses broaden, workplace and environmental safety stay in focus.
A bottle of hexamethyldisilazane (HMDS) doesn’t look remarkable, but it pulls a lot of weight in modern labs and factories. Chemists and engineers often use HMDS to prepare surfaces in the microelectronics world. Stick a wafer in a machine, wash it with HMDS, and suddenly, that surface is ready for action. The stuff acts like a bridge, making sure light-sensitive photoresist sticks during chip-making. Without this preparation step, microchips wouldn’t have sharp circuit patterns. The semiconductor boom of the past few decades owes a silent thank-you to HMDS prepping.
Back in undergraduate labs, organic chemistry assignments would fail constantly if we didn’t dry out our reagents. Water crashes a lot of reactions. HMDS soaks up the last dribbles of moisture, letting reactions run their course. In pharmaceuticals, many life-saving molecules need pure, controlled reactions. Adding HMDS keeps things reliable. Silylation—where a “silyl” group hops onto molecules—runs smoother. These silylated compounds prove easier to handle and analyze, speeding the drug discovery cycle. Factories crank out silyl derivatives for high-performance coatings, adhesives, and special flavors in certain perfumes.
Electron microscopes let scientists peek at bone tissue, bugs, and industrial fibers at levels the naked eye can’t dream about. Biological samples, though, turn into mush when exposed to a vacuum. Treating samples with HMDS helps dry them without damaging their structure. The results jump out in crisp detail. Plant cells, fragile as lace, suddenly become sturdy enough to show off every pore and grain. Research on everything from viruses to advanced materials relies on this step. Without HMDS, a lot of discoveries—especially in life sciences—would get fuzzier, both literally and figuratively.
Anyone who’s handled HMDS remembers the strong ammonia smell and the strict safety warnings. Improper contact irritates skin, lungs, and eyes. Small spills remind you quick that chemical safety training matters. Industries ordering big drums of HMDS know these headaches too well. Spills can slow production and rack up costs. Some research teams have shifted to less hazardous alternatives when possible, but the unique qualities of HMDS keep it in rotation for demanding jobs. Labs mitigate risk with airtight glove boxes, fume hoods, and spill kits at the ready. Proper training and clear rules go a long way. Even so, companies keep an eye on greener substitutes as environmental rules tighten.
People often ask if HMDS counts as high-tech. It really just does a simple task—preparing, protecting, and cleaning. Still, these jobs have ripple effects. From better computer chips to sharper scientific images and glossy coatings on products, HMDS proves that small pieces of chemistry shape huge parts of modern life. Thorough safety habits, honest assessments of risk, and careful sourcing keep this useful tool working for everyone’s benefit. The march of progress relies on recognizing which chemicals make things possible and finding ways to make them safer and more responsible for the next generation.
Most folks don’t use hexamethyldisilazane, or HMDS, in daily life. It pops up more in labs and manufacturing, especially where people prepare silicon wafers or treat surfaces for science projects. Engineers often work with it in chip fabrication. That said, even if it stays behind closed doors, its hazards matter far beyond technicians in white coats.
Ask someone who’s ever caught a whiff of HMDS—the stuff packs an ammonia punch. Eyes water, noses burn. These reactions happen because the vapor irritates the mucous membranes. Prolonged exposure in a poorly ventilated space goes from uncomfortable to outright dangerous pretty quickly. Toxicology data shows inhaling its vapors may cause headaches, dizziness, or nausea. Accidental splashes leave nasty burns on skin, and contact with eyes can be downright damaging.
I remember my grad school days, when maintenance crews brought in drums marked with ‘flammable’ and ‘corrosive’ warnings. The smell escaped even before the lids were off. One day, someone spilled a few milliliters and the whole lab turned into a scramble for fresh air. The lesson stuck: you can’t trust trust your senses to warn you before the real harm hits. The vapors mix well with air and may form explosive compounds. Reports of workplace accidents highlight how quickly things escalate if someone ignores basic precautions or skips that safety training.
Studies published by organizations like the National Institute for Occupational Safety and Health (NIOSH) paint a clear picture. Breathing HMDS can worsen asthma. In high doses, rats experienced lung and liver issues. Human data remains thinner, mainly because most regulations focus on prevention before documentation of actual long-term illnesses. Still, most chemical safety boards urge workers to stay far below recommended exposure thresholds.
Beyond human health, HMDS doesn’t break down quickly. It moves through the air and may end up in groundwater if not handled right. According to the EPA, even small spills can have ripple effects outside the walls where it’s used. Thus, safe disposal and tight containment matter just as much as gloves and goggles.
The story here isn’t all doom and gloom. Labs and factories can keep people safe by sticking to proper ventilation, cutting-edge fume hoods, and personal protective equipment. It helps to train everyone—newcomers and old hands alike—in emergency procedures. Simple habits like storing containers tightly and never pipetting by mouth do more good than fancy posters on the wall. Spill kits and eye washes are not there for looks.
More companies look for safer alternatives when possible or tweak processes to use less of the tricky stuff. Regulation pushes safer design, while open conversations between workers and supervisors prevent shortcuts that lead to disaster. It turns out, respect for what a chemical can do, grounded in real facts and shared experience, is better protection than any warning label. HMDS isn’t going away from industry, but neither are the everyday folks who work with it—so keeping them healthy should always win out over cost or convenience.
Hexamethyldisilazane, or HMDS as chemists call it, often shows up in research labs or manufacturing spaces that produce semiconductors, pharmaceuticals, or specialty coatings. Just glancing at the drum or bottle isn't enough; it's a chemical that commands practical respect. A whiff of its strong ammonia odor already hints at what can go wrong in a sloppy setup. Personally, I've worked in labs where one small spill turned a productive morning into a full-on scramble to flush eyes and vent fumes. Mistakes have consequences. The lesson? Store and handle this stuff right or face avoidable hazards.
A flammable liquid like HMDS wants a cool, dry, well-ventilated home. That means no direct sunlight, no open flames or smoking nearby, and definitely not stashed beside oxidizing acids or bases. I worked in a lab where bottles sat near a radiator—that mistake led to warped lids and nasty evaporation. Fire codes exist for a reason. Flammable cabinet with explosion-proof ventilation worked best in my experience. Metal drum or amber glass container with a tight seal, away from spaeces where a static spark or hot plate could surprise you, gives peace of mind and helps avoid scrambles for the emergency eye wash.
Taking the cap off isn't a two-second job. Don PPE: chemical-resistant gloves, lab coat, splash goggles, and always use a fume hood. Even a tiny splash caused itchy skin for a coworker who forgot gloves once. I never skip the respirator if the job takes longer than a minute. If something does spill, pour absorbent on it immediately and bag everything up as hazardous waste. Don't toss rags or wipes in regular trash. One lab janitor did, and the bin started smoldering half an hour later.
Here’s why you can’t cut corners: HMDS reacts fast with water or humid air and releases ammonia vapor—harsh on lungs, worse for anyone with asthma or sensitive eyes. OSHA and NIOSH recommendations don’t just spell out legal requirements—they save people. Training new staff shouldn’t be a quick slideshow; bring out the physical MSDS, walk through each hazard, and run a demo. Turn on the ventilation fans before uncapping anything, and check the chemical detectors and alarms at least every month. In an old lab I worked in, we caught leaks from loose jug seals only because the monitor pinged right before a night shift walked in.
Regulatory bodies rarely back down from pushing safety. Local and federal inspectors want documented checks, physical labeling with pictograms, and crisis response plans. If your workplace wants ISO certification or insurance peace, track usage and storage logs. I’ve seen insurance carriers pull coverage after seeing rickety shelving or poor labeling on site visits. Skipping protocol invites accidents, legal trouble, and the loss of good people.
Remember, no chemical—especially HMDS—deserves casual treatment. Storing it properly saves money, health, and stress. Training builds confidence and safety culture. Regular checks and careful, label-aware handling means fewer accidents and a stronger team. Treat chemicals with care and they return the favor.
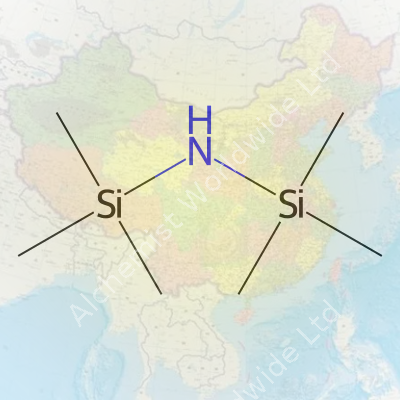
Hexamethyldisilazane — or HMDS, as most chemists call it — shows up in labs and factories more often than most people think. The chemical formula, C6H19NSi2, looks a little intimidating at first glance, but in hands-on use, it’s a tool that solves real-world problems. I remember the first time I handled this compound in a research setting. Its sharp, ammonia-like odor hits you before you even start working, quite a reminder that chemistry is as much about the senses as the numbers.
People who build things out of silicon — think chips and circuit boards — rely on HMDS to make sure surfaces stick to photoresists the right way. The chemical builds a thin layer that preps glass, silicon, and even some plastics so other molecules don’t just peel off later. Skipping this step leads to wasted time, expensive mistakes, or whole batches of electronics heading for the landfill.
Every element in C6H19NSi2 plays a specific role. Six carbons and nineteen hydrogens come together to form two trimethylsilyl groups — each looks like (CH3)3Si–. These sit on either side of a nitrogen atom, which sits squarely in the center. The silicon atoms hold everything together. In everyday terms, the structure lets HMDS act like a shield: it covers up reactive spots on surfaces, making sure water or dust won’t stick around to mess up more precise chemistry later.
Most synthetic chemists keep a bottle or two of HMDS on hand because it helps with silylation, a step that protects fragile groups in a molecule so harsher reactions don’t break anything important. The process saves both time and money, often making it the difference between a successful lab result and a pile of wasted chemicals.
No one should overlook the risks. With all those silicon–nitrogen bonds, HMDS releases ammonia gas when it meets water or humidity. That can cause eye and lung irritation, along with a sharp, pungent smell that lingers in the air. I’ve seen colleagues get careless, only to spend the rest of the day with headaches or worse. Fume hoods, gloves, and goggles aren’t overkill; they’re just practical.
HMDS also presents a challenge for workers in semiconductor fabrication. The chemical can drift through the air if ventilation is poor, exposing workers to that harsh ammonia, especially with repeated use. Building managers need to invest in proper air flow and leak detection, not just checkboxes on a safety audit. Simple changes, like installing better extraction fans or setting strict storage guidelines, go a long way.
Instead of just accepting the hazards, some labs have started experimenting with alternatives, hoping for the same surface magic without the downsides. The technology isn’t quite there yet, but progress tends to happen quickly once engineers and chemists pull together. For now, knowing both the formula and the best practices around HMDS gives workers an edge. It lets them tackle the modern world’s manufacturing problems without stumbling through the same old mistakes.
Hexamethyldisilazane, often called HMDS, pops up in labs for silanizing glassware, modifying surfaces, or prepping samples for electron microscopy. It has a reputation for boosting reactivity and sticking power in science and tech settings. What’s easy to miss is how quickly a splash of HMDS can upend a regular workday. I once watched a respected lab tech duck outside in the dead of winter because one careless moment left them coughing from a single, unseen vapor leak. This stuff cuts through the comforts of routine and lays out the rules—either work safe or risk a trip to the emergency room.
Breathing HMDS fumes does not simply cause discomfort. It burns. Sensation in the nose shifts from sharp to heavy. Dizziness tends to follow. Let enough of it into the lungs, and breathing grows harder. Skin doesn’t get off easily either. HMDS strips away natural oils and irritates even with short exposure. Eyes sting and water for hours after a splash. Ingesting it goes much further, causing burning down the throat and inside the gut. There’s no gentle dose or exception; every encounter with the wrong side of HMDS leaves a mark.
The safest method demands good habits, not just gear. Start with eye protection that wraps around, plus gloves rated for chemicals. Nitrile or butyl rubber keeps hands safe longer than latex. Forget hoodless benches—open bottles or pipettes only inside ventilated fume hoods. Fans outside the hood won't cut it. HMDS vapor carries far, fast, and plays havoc with open eyes, throats, and lungs.
Loose clothing gives chemicals more ways to sneak in. Tuck sleeves, wear lab coats fitted at the wrist, and always keep containers capped or sealed when not using them. Clear labels help, but only if workers actually read and understand them. Stories of mix-ups abound, often because someone trusted old habits instead of reading the label.
Nobody expects an accident, but preparation shifts outcomes. Eyewash stations and safety showers feel redundant until the day they save vision or skin. Colleagues freeze without drills or at least a quick, practiced response. Every lab or shop storing HMDS should run through spill response: rolling up absorbent socks, neutralizing, and then getting rid of contaminated gear properly. Waste disposal centers treat HMDS as hazardous. Pouring even tiny amounts down a drain creates fire risks after the fact.
A dry, cool space away from acids and oxidizers—these aren’t mere recommendations. I’ve seen students return from break to find warped, leaking containers and a frightening cloud of chemical smell. Thick, airtight bottles prevent evaporative losses, so restocking those before starting work saves headaches. Work never starts with faulty containers or unknown leftover amounts.
It makes sense to ask, “Is HMDS the only route?” Some newer treatments now cut down or even skip this chemical for some applications. If the process keeps everyone out of harm’s way, nobody complains about a new learning curve. Reviewing procedures as a team, considering regular updates, and keeping communication open about alternatives keep everyone safer in the long run.
Training beats luck every time. If new staff watch and learn from careful mentors, they carry that on—whether in research, fabrication, or quality testing. A checklist before handling, honest reporting of near-misses, and feedback from seasoned pros stand out as the most powerful shields. Handling HMDS demands more than a casual approach. That diligence, shared across teams, protects careers—and health—every day.

| Names | |
| Preferred IUPAC name | trimethylazane(trimethylsilane) |
| Other names |
Bis(trimethylsilyl)amine HMDS 1,1,1,3,3,3-Hexamethyldisilazane N,N-Bis(trimethylsilyl)amine Trimethylsilylimine Hexamethyldisilazide |
| Pronunciation | /ˌhɛksəˌmɛθɪlˌdaɪsɪˈleɪn/ |
| Identifiers | |
| CAS Number | 999-97-3 |
| Beilstein Reference | 635873 |
| ChEBI | CHEBI:55454 |
| ChEMBL | CHEMBL1544 |
| ChemSpider | 56496 |
| DrugBank | DB14074 |
| ECHA InfoCard | 100.007.318 |
| EC Number | 200-817-4 |
| Gmelin Reference | 71438 |
| KEGG | C06147 |
| MeSH | D006607 |
| PubChem CID | 6517 |
| RTECS number | MK3225000 |
| UNII | VL705UV5CO |
| UN number | UN3380 |
| Properties | |
| Chemical formula | C6H19NSi2 |
| Molar mass | 161.38 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Ammonia-like |
| Density | 0.769 g/mL |
| Solubility in water | Insoluble |
| log P | 0.96 |
| Vapor pressure | 4 mmHg (20 °C) |
| Acidity (pKa) | 25.8 |
| Basicity (pKb) | pKb = 7.9 |
| Magnetic susceptibility (χ) | -45.5e-6 cm³/mol |
| Refractive index (nD) | 1.407 |
| Viscosity | 0.65 cP (25 °C) |
| Dipole moment | 0.60 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 197.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -209 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −5657 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | V03AB38 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H226, H302, H312, H314, H332 |
| Precautionary statements | Precautionary statements for Hexamethyldisilazane: P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P312, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 3-2-2 |
| Flash point | 45 °C |
| Autoignition temperature | 400 °C (752 °F; 673 K) |
| Explosive limits | 1.5–16% |
| Lethal dose or concentration | LD50 Oral Rat 8500 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 850 mg/kg |
| NIOSH | KK1575000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Hexamethyldisilazane: 50 ppm (TWA) |
| REL (Recommended) | Gloves, Chemical goggles, Lab coat |
| IDLH (Immediate danger) | 30 ppm |
| Related compounds | |
| Related compounds |
Trimethylsilyl chloride Hexamethyldisiloxane Trichloromethylsilane |