
Looking at the story of hexamethyldisiloxane, the roots run deep into the rapid evolution of silicone chemistry. Chemists started exploring siloxane linkages around the early 20th century, when pioneering figures looked beyond simple organosilicon molecules to see what unique traits silicon and oxygen can bring to the table. This drive ultimately led to the isolation and commercialization of hexamethyldisiloxane (HMDSO). Back in the 1940s, as industry demand for temperature-resistant lubricants and advanced polymers took off, HMDSO answered the call. Experience in R&D labs has shown that this molecule’s distinct properties bridged the gap between conventional hydrocarbons and more robust silicones. The widespread application in electronics and specialty coatings owes a debt to those trailblazers who first saw the promise in siloxane chemistry.
Hexamethyldisiloxane stands out because it brings together properties rare in both organic and inorganic solvents. Chemists find it in the form of a clear, colorless liquid, carrying a faint, ether-like smell that hints at its volatility. With its unusual blend of silicon and oxygen backbone capped with methyl groups, it slides into niches that demand both hydrophobicity and thermal resilience. Whether you’re handling it in a pharma-grade cleanroom or running experiments in a university lab, its utility holds steady. Regular users see it as a preferred volatile solvent for peptides, a surface treatment for chromatography media, and a protective agent in microelectronics. It’s not just its unique chemical make-up, but its predictable behavior under a range of conditions that makes this product shine in high-value sectors.
Hexamethyldisiloxane comes with a boiling point around 101°C and pours with a viscosity so low it drips like water. Chemically, its resistance to hydrolysis simply outclasses many other silicon-based fluids, provided you keep acids and bases away. Having seen it spill on lab benches, I can say the rapid evaporation lets you clean up without much fuss, though you should never underestimate the need for good ventilation. Its low dielectric constant and compatibility with both polar and non-polar substances give it a versatility you don’t always find among silicones. The methyl-capped structure resists oxidation better than most simple ethers, and its chemical neutrality proves valuable in sensitive analytical and synthetic techniques.
Any reputable supplier prints hexamethyldisiloxane’s assay front and center, usually touting purities climbing above 99.5%. The CAS number—107-46-0—serves as a unique fingerprint. Labels carry warnings about flammability and calls for protective gloves and goggles. Transport containers often come lined or bonded to keep spills contained, since its volatility and flash point around 6°C mean one spark can trigger combustion. In my own experience handling liters of the material, safety data sheets matter as much as the reagent itself, providing shelf-life, storage temperature, and flash-point information that are non-negotiable for compliant use.
Industrially, manufacturers usually synthesize hexamethyldisiloxane by hydrolyzing chlorotrimethylsilane in a controlled aqueous medium, letting the siloxane linkage snap into place as hydrochloric acid drains away. This technical route, forged in the specialty chemicals sector, capitalizes on the energetic driving force of Si–O bond formation. The byproducts—saline wash streams and excess acid—call for downstream waste-neutralization, and process engineers often tweak parameters to maximize yield while limiting environmental impact. In pilot-scale and academic settings, the reaction replicates on a smaller stage, with purification by fractional distillation to carve out colorless, high-purity HMDSO from a tangle of crude siloxane intermediates.
Hexamethyldisiloxane’s chemical life doesn’t stop with simple synthesis; it offers a dynamic platform for further modification. Lab workers regularly deploy it as a precursor in base-catalyzed cleavage, generating trimethylsilanols or even smaller silanols when exposed to strong alkalis. It tolerates gentle oxidation, although more often, chemists value its stability under a variety of reaction conditions. Under the right push, HMDSO participates in C–H bond activation, contributing to the formation of diverse organosilicon derivatives. Surface scientists appreciate how vapor-phase exposure lets it silylate hydrophilic glass surfaces, swapping out reactive silanols for non-stick methyl groups and thus changing physical surface energy. While some might underestimate it as “just another solvent,” those who run sophisticated synthesis see it as more than a bystander—it's an enabler of unique transformations.
Chemists know hexamethyldisiloxane by different names, and switching among them adds flavor to technical conversations. “HMDSO,” “1,1,1,3,3,3-hexamethyldisiloxane,” “bis(trimethylsilyl) ether,” and “O-(trimethylsilyl)trimethylsilane” all refer to the same molecule. Catalog listings might show up under different trade names, depending on the supplier’s branding, but the core characteristics stay the same. In the field, these varying labels sometimes trip up the unwary, so experienced hands always double-check chemical structures before ordering in bulk.
Every handling guide makes one thing clear: hexamethyldisiloxane’s volatility commands attention to ventilation and ignition sources. Training newcomers in the lab, I stress the need for fume hoods, flame-resistant lab coats, and grounded metal containers. The liquid’s low flash point means even static sparks from a poly-blend shirt threaten an uncontrolled fire, so antistatic equipment isn’t optional. Exposure risks focus mostly on inhalation and skin contact; splashes sting, and prolonged inhalation irritates the respiratory system. Industrial sites require regular flammable-gas monitoring and offer evacuation plans tuned to HMDSO’s particular hazards. Regulatory agencies push for closed-transfer systems and robust spill protocols. Waste streams demand incineration or chemical neutralization, reflecting an industry hard-learned respect for environmental stewardship.
Hexamethyldisiloxane earns a place in countless sectors, from pharmaceuticals to microelectronics. Analytical chemists use it to derivatize active hydrogens in complex molecules, boosting volatility for gas chromatography. Electronic manufacturers line up for HMDSO-derived plasma polymer coatings to improve insulation in chip packaging. In the realm of chemical vapor deposition, its breakdown forms thin, conformal films that resist corrosion and heat. Restorers rely on its hydrophobic touch for stone protection, while cosmetic formulators lean on it for making lightweight, water-resistant formulations. Material scientists value the omnipresent molecule for its ability to tune polymer architectures and surface energies, often paving the way for advances in soft lithography or next-gen lubricants.
In the R&D trenches, hexamethyldisiloxane finds new use-cases every year. Researchers investigate novel reaction channels using HMDSO as a protective agent in peptide and oligonucleotide synthesis. My own stints in polymer science labs brought HMDSO into play as a building block for siloxane elastomers, balancing flexibility against the harsh chemical environment. Universities and private labs plunge into studies exploring how this molecule serves as a hydrogen reservoir in catalytic cycles, unlocking lower-barrier reaction routes. Some labs tune plasma deposition parameters to coax unique patterns and functional group densities from HMDSO vapors, hoping to carve out new properties for smart materials or flexible electronics.
Toxicologists have tracked HMDSO’s low acute toxicity in animal models, with lethal doses an order of magnitude above those for alcohol or acetone. The real focus lands on chronic exposure, respiratory irritation, and environmental persistence. Studies point out that—while the molecule rarely bioaccumulates—long-term environmental releases disrupt aquatic life and should stay tightly regulated. Workers exposed over months have reported headaches and minor skin rashes, spurring companies to bolster engineering controls and air monitoring. For communities near production sites, researchers urge continuous monitoring, as even trace levels can trigger regulatory scrutiny. Advances in analytical detection mean even sub-part-billion contamination flags on sensors, raising expectations for cradle-to-grave risk management.
Hexamethyldisiloxane’s story still grows. With the rise of biodegradable polymers and a push toward eco-friendly coatings, innovators see it as a valuable intermediate. Sustainable process engineers chase greener synthesis routes, swapping toxic catalysts and heavy solvents for enzymatic or renewable systems. Next-generation electronics lean heavily on siloxane-derived films, so HMDSO won’t fade from factory floors and research centers. As global regulations tighten on volatile organic compounds, the industry bets on modified HMDSO analogs that combine performance with lighter environmental footprints. Those working at the junction of chemistry, engineering, and environmental science know that HMDSO remains more than a simple solvent—it’s a tool whose continued relevance depends as much on creative innovation as on sound stewardship.
Hexamethyldisiloxane, better known by its shorthand HMDSO, shows up much more often than most folks realize. I first spotted it in a lab during my college years where it stood out for its sharp, almost sweet odor, reminding me of a mix of nail polish remover and silicone spray. It didn’t take long to figure out that HMDSO runs in circles outside of university labs—people who work in electronics, cosmetics, and pharmaceuticals often keep this liquid close at hand.
For chip makers and engineers, HMDSO plays a starring role in creating thin films. Factories that build computer processors use plasma-enhanced chemical vapor deposition—rolls off the tongue, right?—where gases like HMDSO float into chambers and layer by layer, lay down coatings that go into smartphones, tablets, and laptops. Without it, we wouldn’t have those slick touchscreen displays we use every day. I remember a friend in electronics who swore by HMDSO because it helped create reliable moisture barriers. That trick matters, especially as gadgets get smaller and have less room for error.
Most people who swipe on foundation or spritz hair spray don’t think twice about what’s inside the bottle. A look at the ingredients list on personal care products like primers or setting sprays will often reveal Hexamethyldisiloxane. It brings a silky slip to creams and lotions and gives some sunscreen sprays that fine, fast-drying mist. I once tried making homemade lotion and realized the store-bought stuff used ingredients like HMDSO to boost spreadability. Trying to get that glide from just natural oils? Not quite the same result. Big brands choose it because it doesn’t weigh down formulas and helps keep skin smooth without feeling greasy.
Anyone handling precision equipment—like the kind in medical labs or microchip fabrication—runs into HMDSO as a cleaning agent. Unlike water-based cleaners, it dries quickly and leaves behind no residue. A technician I met in a hospital lab preferred it for sanitizing glassware and tools used with sensitive chemicals. By stripping away trace amounts of moisture, HMDSO helps avoid short circuits or contamination. That’s pretty important for people who depend on perfectly sterile conditions for research or diagnostics.
Of course, industry loves a material that pulls triple duty, but that doesn’t give everyone a free pass to ignore its downsides. Hexamethyldisiloxane isn’t especially toxic if used carefully, but large factories can push it into the air during production. Reports from the European Chemicals Agency show some concern about air quality where HMDSO gets used in bulk. Fortunately, tech firms and manufacturers now turn to closed systems and improved filters to trap vapors before they drift outside. People working directly with the stuff should use tight-fitting gloves and solid ventilation, as outlined in OSHA guidelines. Keeping it out of groundwater is just as important. Waste disposal groups in the US and Europe police the dumping of siloxanes, including HMDSO, making sure soil and water stay clean for the next generation.
Green chemistry groups keep busy developing alternatives that do the job without the environmental baggage. Universities continue poking at plant-based and biodegradable options, but HMDSO’s unique balance of water-repellency and low toxicity puts it ahead of the pack in many fields. I expect some start-ups to eventually crack the code, making replacements that satisfy both industry and environmentalists. For now, the responsibility sits with users to follow best practices—protect workers, keep communities safe, and use only what’s needed for the task at hand.
Hexamethyldisiloxane isn’t some rare chemical that pops up once in a blue moon. In labs or in certain industrial shops, it turns up as a solvent, carrier, or part of silicone-based processes. I’ve handled it myself, and it looks harmless at first glance – colorless and on the runnier side. That can be misleading. With a low flash point, this stuff takes off if you give it half a chance and a spark. People get comfortable and cut corners, which almost always causes trouble.
The vapors sneak up on you. Years ago, I worked in a space with poor airflow, and after just a short time, the headache set in. Breathing in the fumes messes with your head, quick. Fire’s another big worry – those same vapors are ready to ignite. The smartest folks I know push for mechanical ventilation from the start. Think about extractor fans, or working under a chemical fume hood. Open windows and box fans just don’t cut it.
Hexamethyldisiloxane fumes love an open flame. Static sparks from clothes, pilot lights, hot surfaces – all fair game. In my experience, nobody thinks their cell phone charger will set off a fire, until it does. Phones, hot tools, light switches: all can provide enough juice for an accident. Store the stuff away from anything plugged in, heaters, or welding setups. Ground yourself and containers if you need to move larger amounts.
Skin burns, eye irritation, lung issues – these crop up from splashes or a lungful of vapor. Disposable nitrile gloves and splash goggles deal with most direct contact. I’ve used lab coats and face shields handling bigger batches. Respirators aren’t overkill if you suspect vapor buildup, especially in cramped quarters. Make sure the gloves and eyewear fit right; loose PPE means trouble.
From experience, plastic or glass bottles with tight caps do the trick. Label everything, even the “temporary” bottles. One technician I knew poured some into a soda bottle just to move it quickly – a classic shortcut that leads to confusion and accidental exposure. Keep storage spaces cool, dry, and out of sunlight. A locked cabinet keeps curious hands out. Never store next to oxidizers or acids.
Spills don’t call for panic, but speed and the right know-how make all the difference. Soak up with non-sparking tools and some form of absorbent, then bag it for hazardous disposal. No sweeping the dust into a corner, no mopping it with bare hands. I keep a spill kit close whenever I break out a bottle. Know where the eyewash and shower stations are in your workspace; practice counts more than you’d think when things go sideways.
Nothing beats hands-on training. Peer-to-peer teaching works best, since stories stick better than lectures. People remember close calls, not the fine print in the manual. Regular reviews and walk-throughs remind everyone what’s at stake: your health and the people working alongside you. Respect for the chemical, paired with good habits, turns risky work into manageable, safe work.
Hexamethyldisiloxane, sometimes called HMDSO, pops up wherever people work with silicones. It has spread through labs and factories alike because of its volatile, water-repelling nature. Glass, electronics, medical devices—all could see some use for this chemical. So, it should come as no surprise that many wonder just what kind of risks this silicon-based compound brings with it.
Breathing any solvent vapor should cause concern, and HMDSO counts as a solvent. Safety data shows that inhaling its vapors for a long time or at high concentrations might irritate the nose, throat, and lungs. Some studies suggest repeat exposure could lead to headaches or dizziness. Direct skin contact can cause dryness or even a rash.
Some organizations, like the European Chemicals Agency, point to HMDSO’s ability to irritate eyes or trigger allergic reactions in a minority of people. Real-world incidents remain rare, but the risks increase without simple precautions—gloves, fume hoods, or solid ventilation.
HMDSO can escape into the air from factories or spill by accident. It evaporates quickly, so outdoors, most of it will drift away instead of sinking into soil or water. That helps reduce certain risks. Still, it doesn't wash away concerns completely.
In the environment, HMDSO doesn’t break down quickly on its own. That means it can linger a bit longer in places without much sunlight or oxygen. In water, it’s unlikely to stick to fish or plants, but it still travels in the air, potentially reaching places far from where it started. I’ve seen how solvents in general can surprise people in their ability to show up downwind—nothing magical protects wilderness from chemicals that ride the breeze.
Regulators keep tabs on HMDSO. In workplaces, agencies set occupational exposure limits. Europe keeps a closer watch than some other places, but even the United States expects labs and manufacturers to keep levels under recommended thresholds. Producers provide safety data sheets with every barrel, laying out steps like "wear gloves" and "avoid breathing vapors."
In consumer products, direct exposure tends to be lower, since finished items usually contain only traces—if that. Still, I know cautious manufacturers screen everything, knowing that chemical traces can cause headaches for the brand, long before anyone falls sick. Trust in safety means managing every step.
Better training, real-time air monitoring, frequent equipment checks—these steps work wonders to stop accidents. Personal experience in busy labs taught me that sometimes the small steps, like fixing leaks and labeling bottles, save the most trouble in the long run. If I could give one piece of advice to workers and supervisors: treat each day handling chemicals like you care about the folks working beside you.
In the end, Hexamethyldisiloxane’s dangers won’t match those of the most notorious toxics, but ignoring it brings unnecessary trouble. Attention, not panic, helps everyone stay safe.
Too many labs treat Hexamethyldisiloxane as a throwaway detail once the drums arrive. Folks lock down the safety data sheets in a drawer, then stack this chemical next to the acetone or leave it in a sunlit corner. The catch: this kind of routine can set up a bad situation. Hexamethyldisiloxane belongs with other volatile and flammable organosilicon compounds. At room temperature, vapors roll out and, if any spark hits, fire kicks up fast. The substance itself doesn’t give a warning through strong smell either, so complacency creeps in.
During my years working with specialty solvents, bad storage came up far more often than it should. Sweat poured off me once while moving leaking drums out of a loading dock; flammable labels half-peeled, no ventilation, rags on the floors. It’s easy to say “follow the manual,” yet daily pressures push people to cut corners. Keeping Hexamethyldisiloxane in a steel or high-density polyethylene container, always tightly closed, prevents evaporation. This is not just box-ticking. Seals must actually fit and work. Otherwise, drips go into the workspace, and vapor can build up—both leading to risks people underestimate.
Direct sunlight heats storage drums, pressurizing vapor under the lid. I’ve seen bulged cans in summer, with surfaces almost too hot to touch. Flammable liquids store much safer in a cool, shaded spot—best is a flammables cabinet or a ventilated storage room, away from any heat sources. This keeps vapor release down and the substance stable. Avoiding temperature swings isn’t a detail. It stops containers from expanding and contracting, which often leads to cracked seals or lids over time.
Hexamethyldisiloxane asks for separation from acids, oxidizers, and open flames. Piling everything close isn’t just lazy—static discharge or accidental spills could trigger combustion or dangerous reactions. I’ve learned to avoid basements prone to moisture or places where people tend to store old rags and demo samples together. Good storage sits on a clearly labeled, metal spill tray, well above floor level, and stays dry.
Stories about surprise vapor build-up, especially on warm days, stick in my memory. Open a cabinet with poor airflow after months of inactivity, and fumes hit you square in the face. Reliable little things—like a fume hood or an exhaust fan—take the guesswork out. Never store this chemical in a closed, unventilated cupboard.
Handwritten, smudged labels disappear after a few wipe-downs. Permanent, printed labels with hazard symbols remove guessing games later. I learned early on that clear labels kept older stock from getting mixed up or used past its best. Every container I’ve seen leak or corrode over the years had one thing in common—no label, or markings too faded to read.
A decent storage plan never comes down to a few locked cabinets. Fire-rated cabinets, regular checks on containers, and keeping extinguishers at arm’s reach make workplaces safer. People tend to leave all this to the new guys or chemical suppliers, but every busy workspace does better by baking safety into daily routines. I’ve been grateful for that redundancy during real emergencies more than once.
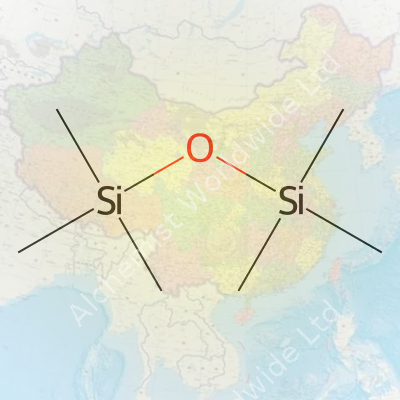
People in chemistry circles call this compound HMDSO, but at its core, it’s a pretty simple molecule. Its chemical formula is C6H18OSi2. If you look at its structure, you’ll spot two silicon atoms joined by an oxygen atom, with each silicon flanked by three methyl groups. Think of it as a bridge, with the oxygen holding the two silicon “islands” together, each decorated with three –CH3 groups. Here’s how it lines up: (CH3)3Si–O–Si(CH3)3.
This setup makes it part of the organosilicon family, which turns out to be a big deal for scientists and manufacturers who want something stable, yet flexible for use in the lab or in big industrial processes. I remember handling it for the first time in a university chemistry lab, impressed that such a seemingly simple structure could show both stable bonds and surprising reactivity.
Every day, researchers and engineers run into issues with contamination, reaction control, or equipment longevity. Hexamethyldisiloxane often winds up as the behind-the-scenes problem solver. People use it as a solvent that resists water, a cleaning agent for delicate electronics, and a building block for bigger, fancier silicones.
This molecule doesn’t just stand on the sidelines. Its strong silicon-oxygen bond gives it solid stability, and methyl groups keep it from mixing with water. Because of this, manufacturers lean on HMDSO whenever water sensitivity threatens a reaction or product. I’ve talked with friends in microchip manufacturing, and more than once, I’ve heard about HMDSO being the go-to barrier for moisture, keeping circuits dry and stable. In healthcare, it provides coatings for needles and other medical devices, reducing friction and infection risk.
No one working with chemicals can afford to ignore safety. Hexamethyldisiloxane, despite its useful features, comes with fire risks and some potential health concerns. It evaporates fast, so good ventilation becomes necessary in workspaces. I’ve seen what happens when spills go unchecked—vapors build up, raising the risk for fire or respiratory irritation. In my own experience, a spill in a closed-off lab took less than a minute before it became uncomfortable. Respirators, gloves, and strict fume hood rules help create a safer space.
On the environmental side, disposal deserves attention. HMDSO doesn’t mix easily with water, which means it can stick around in soil if dumped carelessly. Responsible labs and factories push to recover and re-use it, which protects both the workers and the ground under our feet.
Sustainable practices start with everyone in the lab, from graduate students to longtime technicians. Adopting recycling methods for spent HMDSO, training new staff on safety, and pushing for less toxic replacements or greener processes all help shrink the impact on health and environment. Smaller steps such as reducing unnecessary use and favoring controlled environments have paid off in my own labs, cutting down on both costs and risks. By learning from the way hexamethyldisiloxane behaves, we can keep getting the benefits without passing along trouble to the next generation.

| Names | |
| Preferred IUPAC name | trimethyl(oxo-λ⁴-silanyloxy)silane |
| Other names |
Bis(trimethylsilyl) ether HMDSO 1,1,1,3,3,3-Hexamethyldisiloxane |
| Pronunciation | /ˌhɛk.səˌmɛθ.əlˌdaɪ.sɪˈlɒk.seɪn/ |
| Identifiers | |
| CAS Number | 107-46-0 |
| Beilstein Reference | 1306360 |
| ChEBI | CHEBI:6014 |
| ChEMBL | CHEMBL135312 |
| ChemSpider | 7079 |
| DrugBank | DB14098 |
| ECHA InfoCard | 100.033.418 |
| EC Number | 203-492-7 |
| Gmelin Reference | 110028 |
| KEGG | C06587 |
| MeSH | D006621 |
| PubChem CID | 6617 |
| RTECS number | JM9230000 |
| UNII | G84HK5YIJ4 |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C6H18OSi2 |
| Molar mass | 162.38 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.763 g/mL |
| Solubility in water | Insoluble |
| log P | 2.6 |
| Vapor pressure | 5.5 kPa (20 °C) |
| Acidity (pKa) | 18.0 |
| Basicity (pKb) | 13.6 |
| Magnetic susceptibility (χ) | -45.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.376 |
| Viscosity | 0.65 cP (25°C) |
| Dipole moment | 0.90 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 247.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -576.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4076 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H304, H315, H319, H336, H411 |
| Precautionary statements | P210, P261, P273, P280, P301+P312, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-0-0-F |
| Flash point | 3 °C |
| Autoignition temperature | 210°C |
| Explosive limits | 0.7–11.4% |
| Lethal dose or concentration | LD50 (oral, rat): 8500 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 8500 mg/kg |
| NIOSH | JNMT |
| REL (Recommended) | 0.2 mg/L |
| IDLH (Immediate danger) | 900 ppm |
| Related compounds | |
| Related compounds |
Trimethylsilanol Trimethylsiloxy group Polydimethylsiloxane |