
Iso-octyltriethoxysilane emerged during the push for better adhesion promoters and surface modifiers in the chemical industry. Decades ago, paints, coatings, and plastics faced limits in performance against moisture, weather, and abrasion. Scientists started testing a range of alkoxysilanes and found iso-octyltriethoxysilane married a long hydrophobic tail with a reactive silane head, making it surprisingly adaptable for surface treatment. Suppliers introduced this compound to address the growing needs of automakers, electronics manufacturers, and construction material producers, eager for ways to toughen up their products without major overhauls in processing.
Iso-octyltriethoxysilane stands as a colorless to pale yellow liquid with a faint, nearly alcoholic odor that can be noticed easily during handling. This trialkoxysilane carries a branched C8 hydrocarbon group, lending notable resistance to water and organic solvents. Laboratories and manufacturers lean on its solubility in alcohols, ketones, and hydrocarbons, while it resists dissolving straight into water. Its role revolves around treating glass, minerals, and more, so materials repel water, bond polymers, or gain a boost in electrical insulation.
In the lab, iso-octyltriethoxysilane displays a boiling point around 280°C and a vapor pressure low enough to keep it stable during most processing steps. Its molecular weight hovers close to 320 g/mol, and its density checks in at about 0.88–0.89 g/cm³. The liquid’s reactivity kicks in when exposed to moisture since its ethoxy groups hydrolyze, forming silanols and releasing ethanol. That reaction serves as the starting point for binding the silane to surfaces or crosslinking in polymers. Its hydrophobic octyl group resists water once set, improving anything from construction sealants to protective glass coatings.
Bottles and drums reach buyers with clear markings showing purity, typically above 97%, along with batch numbers and safety labeling according to GHS and local regulations. Production guidelines emphasize limiting moisture and air exposure before application to avoid premature hydrolysis. Manufacturers provide material safety data sheets (MSDS), walking handlers through safe storage at 5–30°C and away from acids, alkalis, and oxidizing agents. The labeling underscores the flammability of the liquid, the potential for eye and skin irritation, and the need for good ventilation.
Large-scale production involves reacting iso-octylchlorosilane with ethanol or a mixture of alcohols, where a suitable catalyst and controlled temperatures ensure full conversion of the chloro group to the desired triethoxy function. In my lab days, this step required tight control over moisture; stray water could generate unwanted side reactions. The byproducts—mainly hydrogen chloride or its salt—were monitored to keep impurity levels low. Modern plants use closed systems to recuperate solvents and streamline purification, helping meet regulatory and environmental standards, while also keeping batch yields high.
This silane’s versatility rests on its reactivity with water or catalysts, sparking the hydrolysis and condensation reactions that let these silanols dock firmly onto inorganic surfaces. After hydrolysis, the silanol groups form siloxane bonds with glass, minerals, or metal oxides. The octyl group faces out, providing a water-repellent cloak. Chemists sometimes modify this backbone to tailor the alkyl chain—or swap ethoxy groups for methoxy—balancing reactivity and process demands. Blending with aminosilanes or methacryloxy silanes can toughen up paints or adhesives, giving those products broader reach in harsh service environments.
Across trade catalogs, iso-octyltriethoxysilane appears under names like triethoxy(iso-octyl)silane, i-octyltriethoxysilane, or even “octyltriethoxysilane.” CAS numbers, typically 35435-21-3, help users track purity and cross-reference supplier offerings. Some firms market it under specialty brand lines, though the core chemical remains unchanged. This helps buyers avoid confusion and check technical data against their needs, especially in regulated applications.
Handling this chemical calls for gloves, goggles, and splash-proof clothing, because even a small amount stings eyes and dries skin. Its vapor can irritate the lungs during spray applications or poorly ventilated work. Chemical plants install well-sealed drum pumps and vapor scavenging to keep exposures down. Fire risk sits in the background, too, since the liquid burns, so fire extinguishers and grounding procedures stay part of standard safety drills. Following REACH regulations in Europe and OSHA in the US, handlers receive annual safety training, regular workplace air checks, and first-aid refreshers for chemical burns and inhalation.
Real-world demand for iso-octyltriethoxysilane comes from concrete sealers, cable insulation, electronics, tough paint primers, and more. Builders treat surfaces with it to keep out water, prevent rebar rust, and stretch concrete lifespan. Automotive engineers work it into underbody coatings to shield parts from road salt and grime. Electronics companies spot-pattern the compound on glass or ceramic, boosting moisture resistance and insulation for sensors or circuit boards. Paint makers focus on scratch resistance, graffiti clean-up, and keeping colors bright in sun, all jobs where the octylsilane backbone performs reliably.
University groups and private R&D labs keep searching for new ways to modify iso-octyltriethoxysilane to freshen up adhesion in plastics, improve fire resistance, or make so-called “smart” self-healing materials. My colleagues tried grafting nanoparticles with this silane, noting boosts in hydrophobicity and durability in outdoor plastics. Research often tests different alkyl groups, blending short-chain and long-chain silanes, to balance processing speed and final product toughness. Scientists also study how surface preparation—glass polishing, pH, curing temperatures—affect silane performance, crafting guides that give manufacturers a practical set of rules, not just theories.
Animal tests and cell studies show iso-octyltriethoxysilane irritates lungs with repeated or high vapors, but stops short of organ-level toxicity at typical exposure levels found in factories or construction sites. Contact tests marked it as a mild skin and eye irritant. Air monitoring in busy workspaces rarely shows values over short-term or eight-hour limits set by authorities. There’s still a need for more data regarding long-term exposure and effects on reproduction or the environment, as the use of silanes expands beyond industrial walls. Regulatory reviews keep pressure on industry to study breakdown products, especially ethanol, and ensure proper waste handling to protect workers and communities.
With more industries calling for greener, longer-lasting materials, iso-octyltriethoxysilane plays a steady role but faces up-and-coming competitors like fluoroalkylsilanes for ultra-hydrophobic needs or functionalized silanes in biomedical devices. Interest grows in bio-based alternatives, but not much matches the performance and affordability of current silanes yet. Companies keep optimizing formulations for water-based processing, faster curing, and lower emissions. If engineers solve issues of volatility, recyclability, and cost, the use of this compound looks set to stretch further—into next-generation electronics, building materials that “heal” their own cracks, and consumer coatings that shrug off stains for years. The intersection of regulatory pressure and technical possibility keeps researchers and manufacturers tuned into safety, sustainability, and rugged performance.
You start to notice how certain chemicals play a hidden but vital role in the things we use every day. Iso-octyltriethoxysilane often flies under the radar for anyone not knee-deep in chemistry, but anyone building bridges, making electronics, or even painting cars counts on it. With a chemical structure that anchors itself tightly to surfaces and then offers one hand to whatever you’re trying to stick, this silane creates bonds others just can’t match. Think of it as strong double-sided tape for a range of materials from glass and metal to rubber or plastic.
In construction, adhesives and sealants rarely get the glory, but nothing hangs together without them. Iso-octyltriethoxysilane changes the game for concrete and glass. Humidity, rain, heat, salt—these conditions tear materials apart over time. Thanks to this silane, though, surfaces grow resistant to water and chemicals. There’s less crumbling, less cracking, less money wasted on repairs. The difference becomes clear if you’ve ever walked across a bridge built in the last couple decades: protection against corrosion owes a lot to chemicals like this one.
Modern electronics feel smooth and seamless, though inside, there’s a battle to keep circuits running clean. The wrong surface treatment can lead to short circuits or failure when moisture sneaks in. Iso-octyltriethoxysilane stands out as a primer in wires, circuit boards, and connectors. Coating these sensitive areas, it acts as a shield to keep out unwanted water and oil—extending product life, cutting back on frustrating recalls and e-waste.
Walk through an auto body shop, or spend time refinishing windows, and you'll see how professional finishers harness this silane. Once added to paints or varnishes, the compound can toughen the layer that sticks to metal, plastic, and even ceramics. Rainwater beads up and rolls off, dirt doesn’t grab hold as easily, and stubborn stains take a back seat. For anyone tired of peeling paint or having to recoat surfaces, it’s a welcome shift in reliability.
Most folks never read a chemical safety data sheet, but serious manufacturers watch toxicity and environmental impact closely. Iso-octyltriethoxysilane already lends itself to lower use rates because of how well it works, trimming down the number of chemicals released into water and soil. Companies can meet stricter health and safety standards without gutting performance—a real win for both workers and the environment. It also helps create finished products that release fewer harmful particles over time, improving indoor air quality and cutting down on sick days or allergies linked to poor workplace air.
Research never stands still. There’s growing demand for even tougher, more sustainable materials in everything from green construction to wearable tech. Chemists keep experimenting, chasing even better ways for Iso-octyltriethoxysilane to play a role—maybe by borrowing from plant chemistry, or by building in safer-by-design practices from the beginning. There’s still work to do, but the basic idea stays solid: strong bonds, better protection, and a bit less waste added to landfills and waterways.
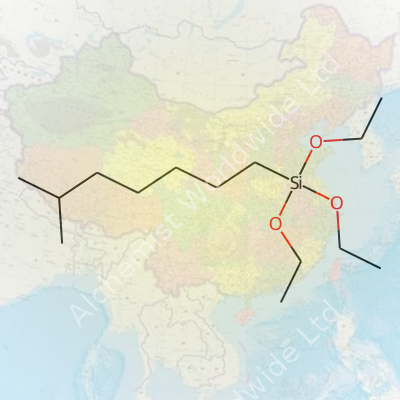
Iso-Octyltriethoxysilane stands out by sporting a branched alkyl chain connected to a silicon atom, which holds three ethoxy groups. Its structure brings to mind clear, colorless liquid, not some chalky powder. The octyl group delivers a big boost in hydrophobic properties—water rolls right off surfaces treated with it. In the lab and on job sites, this quality matters. Getting construction materials to shrug off moisture helps cut down on costly repairs and slow decay.
This molecule keeps its cool until conditions turn moist or acidic. Those ethoxy groups love reacting with water. They swap their ethoxy hats for silanol groups, releasing ethanol in the process. This change turns the silane into a powerful link between organic and inorganic surfaces. On glass, ceramics, or metals, it works like glue, setting up resilient interfaces with added water-repelling powers.
Like many organosilanes, it doesn’t wander far beyond its shelf life if left in containers away from moisture. Give it free access to water, and the hydrolysis runs its course, with alcohols and acids speeding things along. Unopened, containers stay stable for months. Open the cap in a humid room or spill the bottle, and the silane grows sticky fast, loses its clean flow, and eventually gums up equipment. Everyone handling this compound quickly learns not to underestimate humidity during application or storage.
Iso-Octyltriethoxysilane brings more than textbook chemistry to the table. From experience, surfaces treated with it turn markedly more resistant to dirt and graffiti. Wash-off time drops by half or more, especially in city projects. This eases upkeep and reduces detergent and solvent use—a win for anybody who’s spent a Saturday scrubbing outdoor walls.
Emissions matter, too. Compared to harsh fluoropolymers, this silane shows a friendlier side. Released ethanol smells strong but doesn’t threaten environmental health like fluorine chemistry by-products. Still, ventilation is a must; nobody wants to spend hours inhaling alcohol fumes, and eye protection helps against irritation.
Factories benefit by switching to moisture-tight packaging and sturdy seals. Companies often train technicians to prep surfaces carefully, drying glass, stone, or concrete to nudge up the reaction, not kill it with a flood of unexpected water. Simple steps—wiping off sweat from bottles, setting up dehumidifiers in storage areas, and labeling open dates—save time and money down the road.
Some manufacturers blend stabilizers to slow down hydrolysis, pushing shelf life further. These tweaks matter to businesses operating in humid zones, prolonging use and limiting product waste. Research supports what many see firsthand: well-protected containers maintain potency longer. That means fewer failed applications, better performance on the job, and less risk in inventory piles.
Builders face pressure to raise longevity and sustainability, especially under tough weather conditions. Iso-Octyltriethoxysilane steps in, offering a practical middle ground that avoids harsh chemical footprints and supports durable job results. With regulatory standards emphasizing lower emissions and higher safety, knowing the details about storage, application, and chemical behavior isn’t just extra credit—it’s the right move. Genuine know-how about these chemical quirks keeps people out of trouble, protects investments, and raises overall project quality.
Iso-Octyltriethoxysilane pops up in paints, coatings, sealants, and even adhesives. It’s become popular for its water-repelling qualities, which help products last longer and perform better. Still, if you’ve ever worked with chemicals on a factory floor, in a warehouse, or even a small lab, you know that proper storage and handling can make all the difference—to your safety, to your work, and to the stuff you’re trying to protect.
This chemical can irritate your skin and eyes, and it reacts quickly with moisture. Many people in industrial settings, myself included, have seen the mess bad storage can create. Labels fade, drums leak, and humidity sneaks in. These small mistakes invite big hazards—slips, toxic fumes, or worse. Last year, OSHA flagged dozens of citations for unsafe chemical storage, some linked to organosilanes like this one. A barrel leaking in a warm stockroom might seem small, but that’s the start of a much bigger problem. Flooded floor drains, chemical vapors, and emergency room trips add up fast.
Keep this silane out of reach from water, including humid air. People often underestimate how fast ethanol-exuding chemicals can break down when moisture seeps in. Moisture hurts the chemical—turns it hazy, makes containers swell, and, if ignored, can create pressure in drums or bottles. Proper storage means finding a dry, well-ventilated place. I always look for spots where there’s no chance of weather or plumbing leaks. Steel drums and HDPE containers do the trick when tightly sealed, but always check seals for cracks after each use. Never store open containers for “easy access.” Over time, even a loose lid leads to expensive clean-ups and lost product.
Temperature control stands out, too. Silanes start reacting right above room temperature. One overheated storeroom can push vapors into the air—especially risky if there’s limited ventilation. Spills and fumes aside, you’re stuck with ruined material you can’t sell or use safely. Some facilities run temperature monitors with alarms for a reason.
Using gloves and goggles keeps burns and irritation at bay. In my early days on the job, I saw a fellow worker brush aside PPE, thinking “just this once” was fine. A splash to the eyes turned into a lost afternoon at the clinic. It doesn’t take much for serious injury. Proper labeling also goes far with chemicals like this. Clarity on every drum and bottle helps workers grab the right stuff and act quickly during spills. If something does go wrong, spill kits tailored for organosilanes and ethanol come next—preferably on the same shelf or in the next room.
Never pour silanes down the drain or mix with incompatible chemicals. Waste gets managed by specialist teams, and site training keeps everyone sharp. Too many accidents come from workers “making do” or guessing at rules. Regular reminders and updated safety sheets save lives, time, and money.
Supervisors play a hands-on role here. If you’re in charge of a warehouse or production line, check storage areas yourself. Audit the space, look for leaks, ask questions about PPE use, and make sure everyone can find spill kits with their eyes closed. A safety culture beats a fancy written plan every time. Training and honest conversations catch issues early. The goal isn’t to scare, but to make the day go smoother and keep everyone healthy—and that takes careful storage and safe handling, every shift.
Iso-octyltriethoxysilane sits on the ingredient lists of coatings, construction products, and many advanced materials. People often see long chemical names and balk at their content, but nothing beats getting clear on the facts. Designed to add water-repellent properties or improve bonding to surfaces, this silane manages to pop up anywhere from building facades to sealants. The question is—does it create risks at the workplace or at home?
I’ve spent years checking materials safety data sheets before any product touches my shop. The sheets for iso-octyltriethoxysilane paint a mixed picture. Workers who use it in large quantities, or with poor ventilation, face skin or eye irritation. The vapors carry a sharp chemical odor and can cause headaches or dizziness if left unchecked. Handling this compound without gloves or goggles can turn a routine task into a much bigger problem. People who’ve dealt with high concentrations often report rash or itching, echoing the need for real protection, not just good intentions.
On the environmental front, this compound quickly hydrolyzes to produce ethanol and silanols, so it doesn’t persist in the same way as long-lasting pollutants. Still, what comes off as ethanol fumes in a closed space can be quite flammable. Fire risks jump when the chemical drips near ignition sources—even a spark from a light switch can escalate things dangerously fast. Outside, spills can harm aquatic life, especially because the breakdown products lower the oxygen in water.
In shops I’ve managed, strict PPE rules weren’t just lip service. Nitrile gloves, splash goggles, and good mechanical ventilation became standard with silane-based chemicals. Some people assume small batches can do no harm, but skin absorption and vapor inhalation can build up over time. NIOSH and OSHA guidelines specify handling organic silanes in chemical fume hoods or in well-ventilated areas. I’ve seen accidents happen when shortcuts crept in, whether from over-confidence or poor training. Proper storage also plays a role; keeping containers tightly closed, away from heat or flame, avoids most headaches.
For some industries, switching to lower-hazard alternatives gets discussed. The catch: iso-octyltriethoxysilane’s unique properties don’t always have a drop-in substitute. Designers and safety managers often balance risk by limiting how much is used at once, using pre-mixed products where possible, or moving to water-based coatings. From my own experience, if a simpler chemistry can do the trick, that’s the smarter choice. But some performance specs just don’t budge without it, especially for outdoor applications.
What’s worked best isn’t just sticking a manual in a break room, but making training hands-on. If people see what happens to bare skin under a UV light after a spill, or watch a controlled burn test, it drives the point home. Fact-based approaches keep fear at bay and let workers spot issues before they escalate. Everyone remembers the time someone lost their eyebrows to a flame flash or the discomfort of a chemical splash—real stories beat lectures every day.
Iso-octyltriethoxysilane brings value, but it’s not something for casual use. Anyone thinking about working with it should respect its flammability, wear the right gear, and use the best engineering controls available. The worst risks come not from the chemical itself, but from underestimating what a fast-acting solvent or strong vapor cloud can do. For many industries, improvement lies in understanding the hazards, supporting workers with the right gear, and always taking one extra moment to ask, "Is this truly the safest option for the job?"
Many folks outside the chemical world only notice the end result: tougher plastics, glass that holds up against weather, or wires that last years without cracking. Hidden in the process, Iso-Octyltriethoxysilane works like a backstage hand, making sure surfaces match the script laid out by engineers.
My years working alongside industrial teams showed me the fuss over details. When composite manufacturers prepare fiberglass, they deal with surfaces that want to repel polymers. A silane treatment changes the game. A worker sprays or dips the glass fibers into a silane solution. It’s not glamorous. You end up dealing with ventilated rooms, chemical splash goggles, people watching timers like hawks. But that simple bath or spray can take the humble fiber and give it extra strength when molded into sports equipment or car parts.
I watched cable factories handle miles of polymer-coated wires. To keep water from causing breakdowns, they prepare the inner insulation with Iso-Octyltriethoxysilane. By feeding wire through a bath or running it under a spray, they lay down a layer so thin you’d barely see it under a microscope. The silane sticks to the surface, then the outer polymer shell latches on better, and—like that—you have a cable that shrugs off moisture in swamps, jungles, or coastal cities.
In construction, crews use paints and sealers treated with Iso-Octyltriethoxysilane. My experience with building restoration projects showed how brick or stone facades lose their battle against rain over the years. Silane, blended into water-repellent coatings, soaks deep into pores. Once there, it bonds. The treated walls won’t trap water, so you don’t get that freezing, cracking, and peeling cycle in winter.
Some manufacturers run mixing tanks where silane gets added to paint before a painter dips a roller. Others treat surfaces directly. On highways, bridges, or subway platforms, applicators use sprayers to cover bare concrete with silane. Crews move quickly, trying to beat rainy forecasts, because the treatment only works if the surface stays dry for a bit. I’ve watched them—hard hats on, boots caked with dust—making sure every rough corner gets hit. It means fewer potholes where winter salt bites deep.
It’s not enough to talk about what works in a lab. The real test comes out in the factory or on a job site. Every time silane comes out, protocols kick in. Workers rely on ventilation, gloves, and storage that isolates containers from moisture. My experience with industrial EHS teams tells me nobody wants to cut corners—one mistake can bring regulators, plant shutdowns, or worse, sick employees. Attention to responsible handling makes all the difference.
Some critics point to silane’s flammability or the need for solvents. Industry leaders push forward with less volatile formulas and automated mixing systems, aiming to reduce spills or fumes. Research and real-world feedback blend together. Over the years, process tweaks—like in-line dosing or new spray designs—have cut waste and boosted consistency, even as stricter green regulations press the industry to change.
In the end, the real value in using Iso-Octyltriethoxysilane rests with everyone who counts on durable products—whether it’s a fiber-reinforced bridge in my own city or a mobile phone cable stretched between continents. Making these advances practical means working with real-world challenges, keeping people safe, and looking for every chance to do the job better.
| Names | |
| Preferred IUPAC name | triethoxy(6-methyloctyl)silane |
| Other names |
Triethoxy(octyl)silane n-Octyltriethoxysilane Triethoxyoctylsilane |
| Pronunciation | /ˌaɪsoʊˌɒktɪltraɪˌɛθɒksiˈsaɪleɪn/ |
| Identifiers | |
| CAS Number | 2943-75-1 |
| Beilstein Reference | 1851564 |
| ChEBI | CHEBI:87154 |
| ChEMBL | CHEMBL1881111 |
| ChemSpider | 5464445 |
| DrugBank | DB16653 |
| ECHA InfoCard | 03-2119981452-50-0000 |
| EC Number | 012-429-00-2 |
| Gmelin Reference | 1202126 |
| KEGG | C18607 |
| MeSH | D016678 |
| PubChem CID | 115530 |
| RTECS number | ST5502500 |
| UNII | IA91S06113 |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID7020402 |
| Properties | |
| Chemical formula | C14H32O3Si |
| Molar mass | 318.56 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Characteristic |
| Density | 0.88 g/mL at 25 °C (lit.) |
| Solubility in water | Insoluble |
| log P | 3.8 |
| Vapor pressure | 1.2 hPa (20°C) |
| Acidity (pKa) | 13.2 |
| Basicity (pKb) | 13.3 |
| Magnetic susceptibility (χ) | -7.44 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.416 |
| Viscosity | 2.5 mPa·s |
| Dipole moment | 0.87 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 276.76 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | Std enthalpy of combustion (ΔcH⦵298) of Iso-Octyltriethoxysilane: -9240 kJ/mol |
| Pharmacology | |
| ATC code | no ATC code |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| Flash point | 62 °C (Closed cup) |
| Autoignition temperature | 240 °C |
| Lethal dose or concentration | LD50 (Oral, Rat): >2000 mg/kg |
| LD50 (median dose) | > 2000 mg/kg (rat, oral) |
| NIOSH | BY2000000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 200 mg/m³ |
| Related compounds | |
| Related compounds |
n-Octyltriethoxysilane Iso-Octyltrimethoxysilane n-Octyltrimethoxysilane Hexyltriethoxysilane Decyltriethoxysilane Phenyltriethoxysilane Vinyltriethoxysilane |