
Iso-Octyltrimethoxysilane didn’t start out as a lab mainstay overnight. Chemists back in the mid-20th century put in serious work to find organosilicon compounds that could stick to surfaces and last through thick and thin. Silanes caught their eye. As research picked up in industrial and academic labs, the need for better water repellency, adhesion, and durability started pushing boundaries. Blending organic groups with silicon–oxygen bonds at a time when silicone polymers were going mainstream got the ball rolling. Given the push from coatings, construction, and electronics, the tweak of adding an iso-octyl group to trimethoxysilane helped fill some crucial performance gaps—particularly where flexibility, lower volatility, and surface compatibility mattered. Out of years of formulation headaches, robust field testing, and a fair amount of trial-and-error, the chemistry found its foothold.
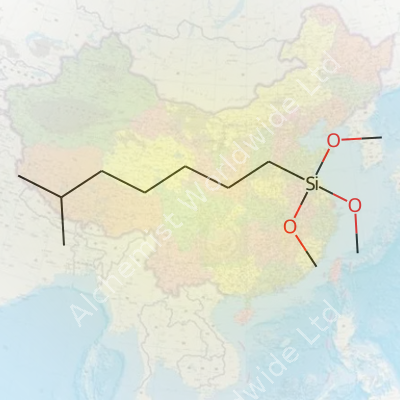
Iso-Octyltrimethoxysilane steps up as a specialty silane coupling agent. In formula, it looks like C11H26O3Si—a compound that’s practically invisible until you see what it actually does. One key strength shows up in its ability to bond organic materials to inorganic surfaces, which matters a lot for the paints or plastics industries. Folk in coatings depend on it for hydrophobic treatments and sticking layers together that otherwise peel or crack. The iso-octyl chain brings flexibility, compatibility, and resistance to the table, making it pop up in textiles, automotive laminates, and even electronics enclosure design. It’s not just another silane; the branching chain provides a unique balance for durability without gumming up a process line.
Iso-Octyltrimethoxysilane usually appears as a clear, colorless to slightly yellowish liquid, with a characteristic organic odor. At room temperature, it eases into a moderate viscosity, making it maneuverable for blending or application by spray or roll. The compound clocks a boiling point just north of 250°C, reflecting the extra heft from the iso-octyl moiety. It flashes at around 90°C, so proper precautions shouldn’t be skipped over in storage or handling. With methoxy groups on three arms, it hydrolyzes quickly upon contact with moisture, releasing methanol—making ventilation and respirator use a mainstay for lab and shop folks.
Supplier labels commonly list Iso-Octyltrimethoxysilane with purity over 97%, sometimes above 99% for high-specification applications. CAS number 34396-03-7 flags it for inventory, while shipping documents highlight its flammability and caution with methanol emission. Most drums and totes get coded with UN1992 for shipping and require GHS compliant hazard labeling—flammable liquid, toxic by inhalation and ingestion, irritant for eyes and skin. Folks needing it at the bench check specs for water content, color, specific gravity (usually around 0.92–0.96), and refractive index (about 1.42) before rolling out production batches.
Industrial manufacturers usually synthesize Iso-Octyltrimethoxysilane by reacting iso-octyl alcohol with trimethoxysilane using acid or basic catalysts, often under inert atmosphere to head off untimely hydrolysis. The process cranks up at elevated temperatures in batch reactors, steering the alkoxylation reaction to completion while keeping side products in check. Methanol released gets distilled off and recycled, and after purification by fractional distillation, the product yields a signature clean fraction meeting technical grade standards. In practice, process flow isn’t simple—moisture exclusion, catalyst selection, and precise temperature control mean plenty of chemists spend more time optimizing than hitting “go.”
The backbone of Iso-Octyltrimethoxysilane centers on those methoxy leaving groups hugging the silicon atom. Drop it into water, and hydrolysis lops off methanol, leaving silanol groups able to crosslink on glass, mineral, or metal. For folks tuning performance, further modifications can add amines, epoxies, or other functional groups to boost adhesion or match target surfaces, pushing its flexibility in downstream synthesis. Some advanced teams play with co-functional alkoxysilanes or combine it with siloxane polymers, driving research in everything from antifog coatings to solar panel encapsulants.
Look around a procurement database or SDS sheet and you’ll spot Iso-Octyltrimethoxysilane under various guises: “n-octyltrimethoxysilane,” “trimethoxy(octyl)silane,” or proprietary names from specialty chemical houses. Overseas suppliers often translate names loosely—examples might include “Octyl-TMS” or “Silane Q8.” The CAS number stays as an anchor for consistency, but crossing between regions or brands sometimes takes a glossary and a patient supplier rep.
Handling Iso-Octyltrimethoxysilane safely means more than just gloves and goggles. Anyone opening a drum or prepping dilution keeps an eye out for volatile methanol off-gassing, especially at higher temps or with splash mixing. Heavy exposure can irritate airways, eyes, and skin, sometimes severely. Long-term exposure to hydrolysis byproducts brings real toxicity concerns, especially for operators on packed production floors. Standard shop protocols recommend splash-proof chemical suits, local ventilation, explosion-proof equipment, regular medical monitoring for chronic effects, and rapid spill containment plans. OSHA and REACH guidance both flag this class of materials as requiring comprehensive chemical hygiene—training, signage, and first-aid kits never collect dust in a responsible operation, because one slip with a silane can mean a trip to the doc.
Iso-Octyltrimethoxysilane gets more real-world action than most realize. Construction supply houses mix it into masonry water repellents and sealants—exterior stone stays dry and free from freeze-thaw crumbling, reducing millions in repairs. Electronics firms favor it in conformal coatings that resist moisture, keep circuits alive, and offer longer lifespans to consumer gadgets. Automotive teams apply it as a primer for composite body parts, boosting adhesion between plastics and paints in wild temperature swings. Textile manufacturers dose it into durable water-repellency finishes, helping outdoor wear brave the elements. Some research labs in solar energy and medical implant fields value its tough, flexible film as a primer or moisture barrier, giving R&D projects a new leg up.
Research on Iso-Octyltrimethoxysilane has shifted from simply characterizing hydrolysis rates or siloxane bonding decades ago to nuanced studies of surface energetics, UV resistance, and compatibility with emerging substrate tech. PhD students and industrial labs in both EU and Asia have probed how molecular tweaks change barrier performance on building materials, or alter interaction with composites designed for wind turbine blades. Many grant projects in renewable energy sources eye silanes for wear-resistant coatings on sensitive photovoltaic panels. Recent years brought machine learning into play, helping chemoinformaticians predict performance when blended with new resins or nanoparticle additives. Collaborations flow across universities, coating suppliers, and auto manufacturers, showing just how far-reaching this one chemical can swing.
Toxicology reports on Iso-Octyltrimethoxysilane flag acute and chronic exposure hazards. Animal studies show that inhalation or skin absorption causes central nervous system effects tied to methanol release. High-dose exposure results in respiratory distress, dizziness, or worse, so workplace limits must be followed to the letter. Environmental scientists report that breakdown products can threaten aquatic life if runoff isn’t managed right. Regulators keep tightening guidelines for exposure, personal monitoring, and containment. Medical researchers continue to track subtle long-term risks, especially where small molecule exposures stack up over years. Even with better PPE and containment tech, the push for detailed health studies won’t end, since chronic toxicity sometimes lurks behind early successful test results.
Talk often circles back to how Iso-Octyltrimethoxysilane will flex in the coming age of stricter green chemistry and sustainability rules. Process engineers look for ways to switch to lower-emission manufacturing, recycle more methanol, and use renewable feedstocks. Formulators chase better biodegradable carriers and greener blends for use with natural stone, recycled plastics, or biocomposites. Electronics makers develop processes that swap solvent-based for waterborne systems, trimming volatile losses. New sensor and semiconductor designers scour surface chemistry for the next magic bullet in device lifespans or environmental resistance. Environmental compliance, circular production, and tighter labeling mean markets will focus on detailed environmental and human health data going forward. Researchers who can crack safer, more sustainable silane chemistry may find a big piece of tomorrow’s industry at their door, proving that lessons learned in the lab and on the shop floor keep paying off.
Iso-Octyltrimethoxysilane pops up in places most people never notice, yet it quietly makes things last longer, stick together better, and resist weathering. That may sound technical, but these benefits have a huge effect on our cars, homes, devices, and even the highways under our feet. I’ve worked on building renovations and seen first-hand how silanes play a role in making coatings stand up to torrential rain and sweltering summers.
This particular silane works as a bridge between organic and inorganic materials. That might sound dry, so here’s what it really means. Manufacturers add Iso-Octyltrimethoxysilane to paints, sealants, and adhesives to help them bond tightly to surfaces like concrete, glass, and metal. Regular paints or sealants often peel, crack, or let water leak through. With the right silane treatment, those products bite into the surface, stay put, and keep working long after standard products fail. It’s the difference between constantly repainting a fence and getting a few extra years before picking up the brush again.
A friend in the auto detailing business explained to me how these silanes get worked into car-care products as well. Windshields treated with solutions containing Iso-Octyltrimethoxysilane shed water, making it easier to see during storms. The hydrophobic properties—basically, causing water to bead up and roll off—has become a selling point for premium glass coatings.
In construction, moisture management keeps buildings standing tall. Concrete absorbs water, which triggers issues like freezing, cracking, and corrosive damage to rebar. Iso-Octyltrimethoxysilane enters the mix as a water-repellent additive. Workers apply silane-based treatments to bridges, parking garages, and high-rise structures. They help block rain and road salts from soaking in, slowing the gradual breakdown of concrete. City governments look for products that extend the service life of major structures, since repairs can take months and blow up budgets. Silane treatments, while not flashy, earn their keep on spreadsheets by holding off the worst of water damage.
Ever cracked open a device and wondered how all those tiny bits stay put? Iso-Octyltrimethoxysilane enhances the performance of shield coatings inside electronics. It lets manufacturers design smaller gadgets without sacrificing moisture resistance or electrical insulation. Experts working on solar panels, LED lights, or even medical gear rely on silanes like this to fight off humidity and keep connections strong.
In an era of heightened awareness around chemicals, safety, and the environment, Iso-Octyltrimethoxysilane stands out for its efficiency at low concentrations. Still, manufacturers and regulators vet the handling and disposal of silanes carefully. Getting trained on material safety and following established process controls keeps workers and communities safe—from proper ventilation during application to innovations like lower-VOC silane systems. I’ve seen tight workplace controls make a real difference in air quality and team health during industrial projects.
Increasing the longevity of everyday materials remains a priority in construction, electronics, and transportation. Adding smart chemistry like Iso-Octyltrimethoxysilane means less waste, fewer repair cycles, and more reliable products. As these technologies advance, the real winners are businesses and homeowners who don’t have to worry so much about what’s going on beneath the surface.
Iso-Octyltrimethoxysilane’s real appeal comes from its silane backbone linked to a branched iso-octyl group. Chemically, the molecule mixes organic and inorganic features—connecting to materials like glass, metal, or ceramics, while reaching out to interact with organics, such as polymers. The silane part features three methoxy groups, which respond with water or moisture in the air. Once hydrolyzed, they turn into silanol groups. These silanols then weave tight chemical bonds with surfaces, almost like a craftsman choosing the strongest glue for a job.
The iso-octyl group avoids water and brings flexibility, not just connecting but adding weather resistance. No matter how much rain or UV light a coating sees, surfaces boosted with this silane dodge early breakdown. The flexibility in the bonds means coatings last, holding up under pressure or temperature swings without splitting or flaking off. It’s not just about protection, though. You get a touch of slipperiness or a lowered surface energy, so dirt, water, and even stains are less likely to stick around.
Straight from the drum, Iso-Octyltrimethoxysilane appears as a clear, low-viscosity liquid. It doesn’t miss a beat, blending into standard organic solvents like toluene or alcohols. Volatility is on the designer’s radar, since methoxy groups can break down, especially if moisture sneaks in where it shouldn’t be. That releases methanol, which can drift into the air. In factories, air quality and worker health matter, so fume control and proper handling aren’t just best practice—they’re basic respect.
Once on a surface, this silane grabs any available hydroxyl groups. Glass has plenty, as does concrete. After applying it, the surface feels different. Touch becomes slick, water beads and rolls off in tight drops instead of soaking in. The chemical change cuts down on corrosion and stops frost or fungus growth. In my experience with stone and concrete, repeated freeze-thaw cycles don’t cause as much damage when this silane is present. Concrete doesn’t crumble as quickly, and stonework shrugs off grime and graffiti more easily.
Every chemist knows that nothing is risk-free. Iso-Octyltrimethoxysilane can cause irritation if splashed on skin or in eyes. Its breakdown byproducts, like methanol, need respect—a ventilated space and gloves guard against hazards. While its chemical strength offers protection, dumping excess into drains leaves lasting problems. Methanol contaminates water, and organosilicon residues can upset aquatic life. I recommend using only as much as needed, then letting leftovers cure fully before disposal.
Iso-Octyltrimethoxysilane holds a quiet reputation among chemists and builders. You don’t often see stained driveways or moss on exposed concrete where it’s been used right. Manufacturers trust the science, and labs back up performance with aging tests and stress trials. Continuous research, like developing processes to cut down on airborne methanol or finding greener alternatives for solvent systems, will push the technology further.
By respecting both the molecular details and the safety realities, cleaners, contractors, and researchers can rely on Iso-Octyltrimethoxysilane to defend and extend what we build. For me, that’s what sets apart a good surface from a great one—one that stays strong and safe for years, not just looks good the day it goes up.
Iso-Octyltrimethoxysilane looks clear, doesn’t have much of an odor, and easily escapes as vapor, so it ends up in the air if you leave it open. In several chemical settings, this compound helps with surface modifications or improves how different materials stick together. The comfort with handling chemicals in the workplace sometimes blinds folks to the risks tucked inside an average bottle. It can really pack a punch if someone breathes in or gets too much on the skin.
With iso-octyltrimethoxysilane, you want a tough, tight-sealing container that resists corrosion. Drums and jugs from high-density polyethylene or stainless steel serve best. Keep these containers off the ground, away from direct sunlight and electrical sparks. Moisture messes with this silane—contact with water breaks it down, producing flammable methanol, so keeping it absolutely dry keeps everyone safer.
Stacking matters, too. Containers stacked too high get unstable and risk tipping. Store drums one high, or at most two, across stable shelving well anchored to floors. Label every container, showing what’s inside clearly, with hazard wording visible. You reduce the risk of anyone confusing it for something harmless.
Stuffing chemicals like this into a cramped closet or backroom never goes well. You want a spot with steady airflow—think a ventilated cabinet or a fume hood, not just a cracked window. Spilled vapors rise fast and spread further than expected, especially in warm climates. Gaps in an old warehouse or a sealed lab might mean the same risk. I’ve seen folks get headaches or dizziness from a one-minute cap-off in a poorly vented storeroom.
Relying on bare hands or fabric gloves counts as a rookie mistake. Butyl rubber, nitrile, or neoprene gloves block this silane’s touch. Eye protection stops splashes. Face shields fill the gap during transfers or decanting. Long sleeves tucked snugly and closed-toe shoes, with good coverage, cut skin exposure down. Respirators fitted with organic vapor cartridges matter in spaces where a spill might ignite vapors.
Open containers slowly to avoid sudden pressure releases. Keep everything grounded during transfers to beat back static sparks—those have ruined more than one lab’s safety record. Absorb spills fast using non-reactive materials, like sand or vermiculite, not sawdust. Refrain from dumping waste in regular trash or sinks; chemical waste drums, clearly labeled and sealed, stay separate. Wash up after each use. I’ve seen experienced workers skip this last bit—skin irritation or breathing trouble follows every time.
No one should handle iso-octyltrimethoxysilane without understanding its dangers. Clear training, regular refreshers, and quick lessons on emergency steps help staff stick to safe habits. Safety data sheets provide real, current info, but plain-language reminders on the wall work even better. Supervisors must check that safety gear never runs short. Workers should always know who to call fast in an emergency.
Even small slips with this compound may lead to fires, injuries, or health damage. I’ve worked in labs where people kept chemicals in plastic bags under benches, and all it took was a few drops spilled, one faulty glove, to send someone to the ER with burns or breathing problems. Sweat the details: dry, labeled containers; protective clothing; smart teamwork. Safety is not just a box to check off, but a daily discipline—acting before anything goes sideways proves you care about yourself and your coworkers.
Few things ruin a surface faster than moisture creeping where it doesn’t belong. In construction, bridges and buildings last longer if water can’t seep into the structure. Iso-Octyltrimethoxysilane steps in as a reliable shield. Applied to concrete, bricks, or tiles, it forms a tough, chemical bond at a molecular level. Instead of surface paint that flakes with time, silane treatments block water and stains right at the beginning. Roads treated with this compound keep salt and moisture at bay, which leads to fewer potholes and less repair work. Clients in the restoration business swear by it because protected masonry doesn’t crumble under freezing rain or snow.
Flaky paint looks cheap and sends folks running for the hills. Iso-Octyltrimethoxysilane acts as a “bridge” between mineral surfaces and organic coatings. Manufacturers add it to primers and paints for metal, glass, or stone. The difference is clear: surfaces hold color longer, scratch and chip less, and shrug off graffiti with less effort. Protective coatings on marine vessels often include this silane so the hull repels barnacles and resists salty spray. Even high-end automotive coatings use it because drivers notice when a car stays clean after a rainstorm.
Modern plastics seem slick, but sometimes their chemical resistance makes them hard to work with. Iso-Octyltrimethoxysilane steps up here as a coupling agent. In fiberglass and plastic composites, it helps the fibers grip the resin, reducing weak spots that lead to cracks. This extra strength is critical for wind turbine blades, aerospace panels, and watercraft parts. These industries demand reliable performance, especially where vibration or weathering cause regular adhesives to fail.
Electronics lead hard lives—humidity, dust, oil, and sweat cause devices to fail at the worst times. Smartphone touchscreens need treatment to keep fingerprints and moisture from causing glitches. Iso-Octyltrimethoxysilane fits in anti-fog and anti-smudge coatings for gadgets ranging from fitness trackers to car infotainment panels. As microchips get smaller and more tightly packed, a single drop of moisture can mean disaster. This silane makes a protective barrier that gives electronic assemblies a fighting chance.
More industries demand green chemistry and safer workplaces. Solvent-based repellents often come with health and pollution risks, so companies look for alternatives. Iso-Octyltrimethoxysilane offers low toxicity, low volatile emissions, and consistent results. While powdered silanes sometimes release dust or fumes, iso-octyltrimethoxysilane handles easily in liquid form. Workers like a product that doesn’t stink up the factory or cause rashes, and labs appreciate less environmental hassle from cleanup.
Construction, coatings, electronics, and advanced polymers all benefit from the clever design behind iso-octyltrimethoxysilane. It doesn’t just sit on top of a surface; it becomes part of the material. My own hands-on experience with moisture-damaged concrete taught me the value of silane technology—weatherproofing done right means less money spent fixing things down the road. Smarter surface chemistry lets builders, manufacturers, and designers work with confidence in both performance and safety.
Iso-Octyltrimethoxysilane pops up in plenty of places, from making specialty coatings to waterproofing sprays. Most people won’t stumble across this word at the grocery store, but plenty of workers in factories and labs handle it every day. This chemical helps things stick together, keeps surfaces water-resistant, and acts as a “linking” agent for things like glass or metal in paints and sealants.
No one likes a long ingredient list with words that don’t seem familiar. Iso-Octyltrimethoxysilane carries some real risks. Inhaling the vapors or getting this chemical on your skin can irritate the nose, throat, eyes, and skin. People who breathe in dust or fumes at work—if their masks slip or ventilation breaks—often report headaches, coughing, or red eyes. There’ve been cases where folks working around silane compounds developed allergic skin reactions or even chemical burns when safety rules got skipped.
The bigger long-term health risks are less clear. Right now, there’s not a pile of studies showing cancer or big chronic diseases linked to occasional exposures. Still, the Occupational Safety and Health Administration (OSHA) and the National Institute for Occupational Safety and Health (NIOSH) both recommend protective gloves and masks as standard practice. Working with chemicals like iso-octyltrimethoxysilane day in and day out could take a toll over time, especially if protective gear gets ignored.
ISO-Octyltrimethoxysilane doesn’t stay in the lab. It moves with wastewater and can leach into the air at production plants. The main worry comes from its tendency to hydrolyze—meaning, it reacts with water in the air or soil and can turn into methanol, a known hazardous substance. Methanol’s not just toxic to drink; it can also hurt wildlife when it seeps into groundwater. Since it doesn’t break down overnight, it sticks around long enough to make a dent in water quality.
From my years around industrial chemistry, most problems don’t come from the chemical itself but from how we handle it. Companies are pressured to keep emissions low, pay for waste cleanup, and dodge big fines by tightening up everything from air filters to staff training. Engineering controls like sealed systems go a long way. Without decent education and safety audits, short cuts become accidents.
Safer alternatives and green chemistry push industries to check for less hazardous chemicals wherever possible. Some newer organosilanes take the place of iso-octyltrimethoxysilane and behave better in the environment, breaking down faster so groundwater and soil aren’t hit as hard. The EPA keeps a close eye on chemicals entering the manufacturing world and flags those that threaten workers or communities. Companies with a long view invest in products with solid toxicity profiles—not just to avoid laws, but to keep trust with their customers and neighbors.
Open communication works better than secrecy. Plants with good reporting and community involvement let people know about what’s flowing out their back doors. Health and safety experts encourage routine checks, air monitoring, and safety briefings. It’s not about banning chemistry, but about making smart, responsible decisions.
| Names | |
| Preferred IUPAC name | trimethoxy(6-methylheptan-2-yl)silane |
| Other names |
Trimethoxy(octyl)silane n-Octyltriethoxysilane Octyltrimethoxysilane Isooctyltrimethoxysilane Trimethoxy(iso-octyl)silane |
| Pronunciation | /ˌaɪsoʊˌɑːk.tɪl.trɪˌmɛθ.ɒk.siˈleɪn/ |
| Identifiers | |
| CAS Number | 107-46-0 |
| Beilstein Reference | 3528173 |
| ChEBI | CHEBI:88374 |
| ChEMBL | CHEMBL4292380 |
| ChemSpider | 192106 |
| DrugBank | DB16655 |
| ECHA InfoCard | 100.168.786 |
| EC Number | 2768-02-7 |
| Gmelin Reference | 1161268 |
| KEGG | C19440 |
| MeSH | C09-463 |
| PubChem CID | 85755 |
| RTECS number | TI4376000 |
| UNII | E2D01GDZ8C |
| UN number | “UN1993” |
| CompTox Dashboard (EPA) | DTXSID3020702 |
| Properties | |
| Chemical formula | C11H26O3Si |
| Molar mass | 292.52 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.88 g/cm3 |
| Solubility in water | Insoluble |
| log P | 3.93 |
| Vapor pressure | 0.4 hPa (20 °C) |
| Acidity (pKa) | 13.0 |
| Basicity (pKb) | pKb: 5.5 |
| Magnetic susceptibility (χ) | -6.52×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.421 |
| Viscosity | 1.0-2.5 cP |
| Dipole moment | 1.24 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 720.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H411 |
| Precautionary statements | H226, H315, H319, H335, P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P370+P378, P403+P235, P405, P501 |
| Flash point | 75 °C |
| Autoignition temperature | 270 °C |
| Explosive limits | Upper: 6.9%; Lower: 1.1% |
| Lethal dose or concentration | LD50 Oral Rat: >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Iso-Octyltrimethoxysilane: Oral, rat: > 2,000 mg/kg |
| NIOSH | Not listed |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 100 mg/m³ |
| Related compounds | |
| Related compounds |
n-Octyltrimethoxysilane Iso-Octyltriethoxysilane n-Octyltriethoxysilane Methyltrimethoxysilane Vinyltrimethoxysilane Phenyltrimethoxysilane |