
Methacryloxymethyltriethoxysilane has grown into a critical component of modern materials science. When people first started experimenting with organosilanes in the twentieth century, the attention landed on their ability to form bonds with both organic and inorganic surfaces. The drive for stronger adhesives, coatings that stand up to weather, and composites that don’t crack so easily pushed chemists to dig into new monomers. Methacryloxymethyltriethoxysilane emerged from these experiments during a period of rapid innovation in the 1970s. By marrying a methacryloxy functionality—well known from acrylic resins—to a silane backbone, researchers created an ingredient that anchors to glass and minerals but also reacts with resins. That opened the door for industries like automotive or electronics, where engineers always look for ways to make plastics, rubbers, and glass play nice together.
Few additives compare to methacryloxymethyltriethoxysilane in versatility. The molecule acts as a silane coupling agent, which means it can graft onto silica-rich fillers, pigments, fiberglass, or even some metals. Given the methacryloyl group sticking out, this silane plays well with unsaturated polyester, acrylate, and methacrylate polymers. Users end up with improved bonding at the interface between the resin and the inorganic component. The market knows this chemical not only as a coupling agent, but as a primer, surface modifier, or crosslinker, depending on who uses it and what demands the process makes.
Methacryloxymethyltriethoxysilane presents as a clear to light yellow liquid, with a distinct sharp, ester-like odor that signals reactive groups ready to participate in condensation or polymerization. It runs with a specific gravity close to 1.05 at room temperature, which makes it just a touch heavier than water. The substance remains stable under ordinary storage, but its triethoxy groups hydrolyze in the presence of moisture, forming silanols in the process. The methacryloyl group at one end means the molecule tacks on to polymers via free radical mechanisms. Chemists value the dual reactivity: alkoxy silane hydrolyzes and condenses with inorganic surfaces, while the methacryloyl group participates in cure reactions with organic matrices.
On a typical technical data sheet, methacryloxymethyltriethoxysilane carries purity levels above 98%, not just for transparency, but because any residual methacrylic acid or ethanol can interfere with product performance. The CAS number for this substance—often cited as 2530-85-0—serves as a universal reference across suppliers. Regulatory bodies such as OSHA and the European Chemicals Agency expect clear hazard and precaution statements, since improper handling may release methacrylic acid or ethanol vapors. Bottle labeling lays out recommendations for storage below 30°C, away from sources of moisture, and calls for sealing the container tightly each time. Packaging often consists of HDPE drums or glass bottles to avoid unwanted chemical interaction.
Manufacturers rely on a synthesis route that starts with chloromethyltriethoxysilane and methacrylic acid. The two feedstocks react under controlled conditions in the presence of a base—like pyridine or triethylamine—as a catalyst. This process gives off hydrochloric acid as a byproduct, which the operator scrubs or neutralizes before purification. Reducing moisture and oxygen exposure during the reaction prevents premature polymerization of the methacryloyl group. The final crude product gets distilled under reduced pressure to remove unreacted starting materials and byproducts, producing a substance suitable for use in high-performance resin systems.
With two reactive ends, methacryloxymethyltriethoxysilane stands out in its class. On contact with water, the triethoxy groups hydrolyze, yielding silanol groups that bond to mineral surfaces or generate crosslinked siloxane networks. In composite manufacturing, these silanols create a tenacious link with glass fiber or silica-filled resins. Chemists use this chemical’s methacryloyl group to co-polymerize it with acrylates, methacrylates, or styrenics, building hybrid networks. Variants with longer alkyl spacers or modified side groups sometimes see use in specialized resin or elastomer blends, where improved water resistance or modified flexibility is in demand.
The marketplace knows methacryloxymethyltriethoxysilane by a suite of alternative names. Some chemists call it MEMO-silane, a term that sticks in laboratory conversations across North America and Europe. Product catalogs may also list it as methacryloxypropyltriethoxysilane or by its trade names, which large chemical producers use for branding—names such as Dynasylan MEMO (Evonik), KBM-503 (Shin-Etsu), or Silquest A-174 (Momentive). This naming convention helps buyers match existing customer certifications, streamline approval processes, and access relevant safety data.
Responsible handling of methacryloxymethyltriethoxysilane demands a focus on safety from the loading dock to the process line. The liquid irritates the skin, eyes, and respiratory tract, especially in poorly ventilated or confined areas. Process engineers stay alert to the flammability of both the neat liquid and the methacrylic acid or ethanol vapors released during hydrolysis or cure. Plant workers suit up with gloves, goggles, and chemical splash aprons, and many companies install local exhaust hoods or scrubbers near mixing or application points. Standard operating procedures call for immediate cleanup of spills with inert adsorbents and safe disposal by trained personnel, minimizing the chance of exposure or accidental contamination.
Methacryloxymethyltriethoxysilane finds broad use across dozens of industries. Composite resins for boats, automotive body panels, and wind turbine blades rely heavily on this silane when reinforcing glass fiber with unsaturated polyester or vinyl ester matrices. Electronics manufacturers draw on its coupling ability to prime glass or silicon wafers before applying dielectric or adhesive layers. In coatings, the chemical helps paints and surface treatments grip tightly to concrete, ceramics, or metals. Dental material suppliers use it to prepare glass or ceramic fillings for bonding to acrylic resin, while medical device makers trust its low toxicity after cure. Wherever a tough, water-resistant bond between organic and inorganic materials pays off, this chemical shows up as a workhorse.
Scientists keep looking for new ways to enhance the performance and user safety of methacryloxymethyltriethoxysilane. At universities and R&D labs, studies focus on the interface chemistry between this silane and next-generation nanoparticles, nanofibers, or functional fillers that power flexible electronics or smart packaging. Researchers dive into how structural tweaks—such as branching, chain length, or fluorination—change the weathering or chemical resistance in finished parts. Collaborative projects between academics and industry have produced silane blends with improved hydrolytic stability or tailored activation times that help manufacturers slash waste and cut energy bills. This constant push for improvement underscores the chemical’s essential role in sustainable, high-performance materials.
Long-term exposure studies in rodents and cell lines gave scientists confidence that methacryloxymethyltriethoxysilane, once fully cured or polymerized, poses low toxicity risks to users and end consumers. Short-term exposure can still produce acute skin and respiratory irritation, so industrial hygienists emphasize proper ventilation, protective clothing, and rigorous training for staff. Environmental chemists have reported that, when hydrolyzed in wastewater or soil, the breakdown products mirror those of other alkoxysilanes and methacrylate monomers, without persistent or bioaccumulative traits commonly linked to more hazardous industrial chemicals. Regulatory authorities in the United States, Europe, and Asia have set workplace exposure limits and disposal guidelines to protect workers, communities, and the environment from any accidental releases or chronic low-dose exposure.
Looking ahead, the demand for lightweight, corrosion-resistant materials keeps the spotlight on silane coupling agents like methacryloxymethyltriethoxysilane. Green chemistry initiatives are pushing both established producers and new startups to explore renewable feedstocks and cleaner, solvent-free processes for making this chemical. Applications linking composite reinforcement with electrical conductivity or responsive surfaces for diagnostics and wearable tech are expanding. Researchers develop new derivatives that survive harsher environments, such as deep-sea pipelines, satellites, or medical implants. As construction, transportation, and electronics all chase higher reliability and reduced carbon footprints, methacryloxymethyltriethoxysilane stands out—not just for what it contributes today, but for the creative ways it’ll anchor tomorrow’s breakthroughs.
Building better materials often means finding the right way to help things stick together. Methacryloxymethyltriethoxysilane, despite its long name, has a pretty straightforward job. This silane acts as a bridge between stuff that doesn’t naturally mix, like plastics and glass or metals and synthetic resins. It grabs onto both surfaces and helps create stronger, more reliable bonds.
Back in my time working in research labs, I’ve seen the headaches caused by weak adhesion. Composites would peel apart, coatings would flake off, and everything from circuit boards to car bodies lost their shine fast. By introducing silanes like methacryloxymethyltriethoxysilane into the process, those issues dropped dramatically. The chemical latches onto different surfaces because it carries groups that react with both inorganic and organic materials. Basically, it gives engineers and chemists more control over how materials hang together under stress, heat, or moisture.
Car makers and aerospace engineers have always chased lighter, tougher, smarter materials. Adding silane coupling agents makes a difference in how fiberglass stands up to the weather and the rough treatment roofs, vehicles, or wind turbine blades take every day. Silanes help move strong fibers and stiff fillers into new kinds of plastics, so products don’t just look sharp—they last longer on the job.
Paints, adhesives, and sealants need to stay put after they’re applied, not peel or crumble as soon as things heat up or get wet. That’s where methacryloxymethyltriethoxysilane steps in. It creates chemical bonds within resins that block water and keep layers stuck in place, which cuts down on repair bills and waste. My firsthand work with water-resistant coatings for outdoor signs showed that adding the right silane cut down on weathering, which meant brighter, more durable finishes for years, not months.
People who handle chemicals like this need up-to-date safety info. Methacryloxymethyltriethoxysilane can irritate skin and eyes, and strong fumes may cause trouble in a workshop if proper ventilation slips. Labs and plants use gloves, goggles, and hoods to keep workers safe. Good storage routines and careful cleanup stop problems before they start. Reliable studies show that these practices sharply cut health risks compared to old-school, lax habits.
Sustainability has picked up speed in just about every industry. Using methacryloxymethyltriethoxysilane in recycled plastics or eco-friendly paints shows a way forward. It builds tougher products, so they don’t need replacing as fast. Reducing waste and boosting the lifespan of materials helps companies lower costs and shrink their environmental impact. I’ve worked with teams reusing glass fibers in construction panels, and the right silane made the recycled mixes just as tough as those made from new materials.
Innovation depends on details, and methacryloxymethyltriethoxysilane lives in the fine print of better products. Its ability to connect worlds that don’t naturally get along means smoother, tougher, longer-lasting materials. That translates into stronger bridges, lighter vehicles, and buildings that hold up longer—stuff that really matters, far beyond the lab bench or the manufacturing line.
Methacryloxymethyltriethoxysilane plays a valuable role in the formulation of coatings, adhesives, and composites. Many folks working in labs or manufacturing mix this compound into vinyl ester resins, sealants, or fiberglass settings. Its chemical structure gives it a reactive edge, but at the same time that edge can bring risks if someone overlooks the basics in storage and handling.
Air and moisture both cause trouble for this silane. Leaving containers open or stored in humid spaces often leads to hydrolysis—once exposed, it turns cloudy, loses performance, and becomes harder to manage safely. A dry and cool spot will keep this liquid in good shape. I’ve seen cracked containers and ruined batches, and usually the problem traces back to hot warehouse racks or careless storage near doors where temperature and humidity jump all over the map.
Anyone with a drum of this silane lying around should rely on tightly sealed, corrosion-proof packaging. Steel with a specific coating or HDPE jugs slow down contamination and stop leaks. I’ve found that far too many accidents in storage rooms come from mismatched packaging, leaky caps, and letting incompatible containers clutter up the shelf.
This liquid will react with strong acids, bases, and oxidizers. Acidic vapor in the same room will attack the silane. Mixing mistakes or just placing incompatible chemicals nearby sets up a cocktail for runaway reactions or ruined stocks. I always parked methacryloxymethyltriethoxysilane away from acids, even if it meant installing an extra storage locker. It’s cheaper to set up a separation barrier than deal with an emergency spill.
Vapors can irritate skin, eyes, and lungs. Gloves, goggles, and sturdy lab coats aren’t optional. Even after years using these materials, I’ve seen colleagues get complacent, skip eye protection, and end up with chemical burns or respiratory issues. It only takes one carelessly handled pour to cause weeks of recovery. Workstations need local exhaust fans and well-placed spill kits to absorb leaks or accidental splashes.
I always recommend a safety shower nearby since rinsing right away limits damage from exposure. Emergency response launches smoother when everyone knows where these stations sit and how to use them. Regular safety drills matter because real spills spill don’t give anyone time to scroll through the manual.
Unused portions never head down the drain. Waste teams use proper labeling and tightly closed secondary containers. Fire marshals, environmental health experts, and waste contractors love clear documentation — and so does the company’s bottom line, since cleanup for unauthorized dumping or leaks brings sky-high costs and fines. Vehicles transporting this material need good ventilation, and drivers check seals and fix tie-downs before moving a drum. Many companies use temperature monitors to track loads during summer to make sure the silane isn’t cooking in the back of the truck.
No one likes surprises in storage rooms: a little planning goes a long way. Assigning responsibility for weekly checks, writing down opening dates, and using up older stock instead of new drums stops waste and preserves quality. In my experience, the companies that avoid incidents treat chemical storage like grocery inventory—first in, first out, tightly tracked, and never neglected until the tank runs dry.
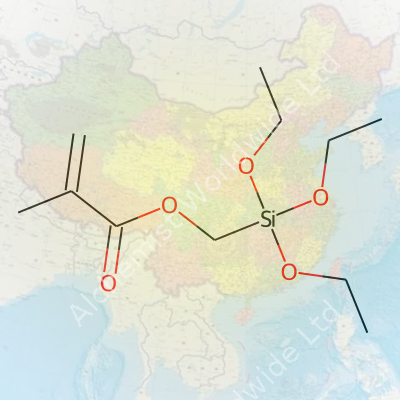
Methacryloxymethyltriethoxysilane, known in research circles for its role in advanced materials, carries the CAS number 2530-85-0. Its chemical formula: C11H22O5Si. The molecule brings together a methacrylate group, a methyl bridge, and a silane core capped by three ethoxy groups. Its structure: CH2=C(CH3)COOCH2Si(OC2H5)3. Each fragment shapes how this hybrid molecule reacts—in labs, in manufacturing, or out in the field.
This silane isn’t just a curiosity for chemists. Its unique dual-functionality lets it connect organic polymers to inorganic surfaces, an advantage that’s hard to overstate. I've seen it star in composites, feldspathic ceramics, and coatings. In dentistry, the same chemistry that keeps a filling in place also supports strength in specialty adhesives for composite bridges. From paint manufacturers to electronics makers, many have leaned on its structure to solve problems ordinary binders can’t touch.
Importantly, you end up with bonds that last. The methacrylate group takes to common acrylic monomers, locking into cross-linked networks during curing. The triethoxy silane head, on the other hand, lets it latch onto glass, ceramics, or metal oxides through hydrolysis and condensation. This tightly-knit bond resists peeling and delamination, boosting durability even in rough conditions—something I’ve seen firsthand on gear exposed to moisture and UV for years.
The same chemical properties that give methacryloxymethyltriethoxysilane value also require a thoughtful eye during production and use. Ethanol sits among the volatile byproducts during hydrolysis. Silane vapors can irritate airways and eyes if workers don’t have the right protection. In my experience walking plant floors, clear labeling, ventilation, and ongoing training turn safety from a box to check into a culture. It’s not about scaring workers; it’s about giving them the facts and the gear to act smart.
Disposal can’t be an afterthought, either. Leftover silane and contaminated containers call for collection and treatment—dumping down drains or into landfills damages trust, harms water, and invites legal headaches. Solutions can fit the scale of use: from local solvent recycling for labs to full-fledged hazardous waste partnerships at large facilities. Proper routes matter—methacrylate’s not benign in water systems and the silane fragment can linger in the wrong environments.
Trying to cut corners with substitutes promises savings but brings headaches. Buyers sometimes don’t see the difference until a batch fails, wrecking composite strength or sending coatings peeling. Over the years, I’ve watched manufacturers turn to testing standards—infrared spectroscopy, GC-MS, and NMR—not as red tape, but as insurance. Full COA (certificate of analysis) paperwork, lot tracking, and transparency put everyone on the same page. Your production manager, the lab down the street, and the final customer all benefit when no one needs to guess about purity or reactivity.
The structure and CAS number for methacryloxymethyltriethoxysilane aren’t just trivia for data sheets. Knowing exactly what’s in play builds trust—from ongoing research projects to manufacturing lines putting out ton after ton. Sharing this information lets innovation do its job and accountability thrive. Only with clear, accessible data can teams base decisions on evidence, not hope or habit, and move chemistry forward while respecting both safety and sustainability.
Most folks won't hear much about methacryloxymethyltriethoxysilane unless they're deep into the world of plastics, coatings, or adhesives. This chemical sounds like the technical jargon reserved for the lab, although traces of it end up all over modern manufacturing. It's mainly used as a coupling agent, linking materials so products stay durable and weather-resistant. But with chemicals, the conveniences of modern production need an honest weighing of possible risks, especially for workers and folks in nearby communities.
Run into methacryloxymethyltriethoxysilane on a production floor or through a shipping spill, and trouble can follow. Most safety data sheets warn that direct contact with skin or eyes stings and can leave redness or burning. Inhaling vapors could irritate the nose and throat, and swallowing small amounts brings stomach issues. So basic PPE—gloves, goggles, and decent ventilation—still stands as the daily armor.
We know from reports and industry guidelines that this silane isn’t quite as nasty as its more famous cousins, like formaldehyde or benzene, which workers connected with higher cancer rates. Short exposures often cause acute problems rather than long-term disease. This doesn't mean it's harmless, just that immediate care often keeps people out of real danger. But, as with many chemicals, repeated sloppy handling might stack up to something worse.
Worries don't stop with workplace accidents. Over time, small spills or poor ventilation piles up. In poorly run facilities, residue gathers on surfaces or escapes into wastewater. Methacryloxymethyltriethoxysilane breaks down into methacrylic acid and ethanol, both of which come with their own hazards. In the air and water, this breakdown can add to local pollution, hurting aquatic life.
Research on chronic exposure remains pretty thin. Long-term animal studies haven't flagged major devastation, but most responsible chemists agree that what holds for rats in the lab doesn't guarantee full safety for people. Modern rules, especially in places like California under Proposition 65, encourage companies to limit worker exposure and track emissions closely. Regulatory bodies like OSHA and the European Chemicals Agency demand serious paperwork and regular risk assessments, not just a waiver sign-off.
Most hazards tied to methacryloxymethyltriethoxysilane come down to how and where it's handled. Factory workers on the front lines need clear training and access to real PPE, and companies have a duty to install extraction hoods and leak detection. For towns near big manufacturers, tighter spill controls and good neighbor policies ease worries.
Safer alternatives in industrial chemistry tend to cost more. Still, if a plant wants to last for decades, it only stays open by keeping both its staff and neighbors out of harm’s way. Third-party audits help, as do tough penalties for willful neglect.
At the end of the day, every chemical used in manufacturing carries some risk. Methacryloxymethyltriethoxysilane deserves careful handling and regulations grounded in real-world evidence. That respect for facts protects people’s health, strengthens trust, and encourages innovation that cares about communities as much as it does about profit.
Chemistry doesn’t always seem personal, but most of us who spend time in labs have ended up with a strong opinion about silane coupling agents. The expectation for a silane like methacryloxymethyltriethoxysilane is pretty clear: act as a bridge between inorganic fillers and organic resins, improve bond strength, and keep coatings or composites from crumbling under real-world conditions. Too often, folks just toss in a dose and hope. From what I’ve seen, a little planning goes a long way.
It’s tempting to dump this silane straight into a batch and move on, but the outcome rarely shines. Silane chemistry depends on moisture — ethoxy groups hydrolyze, releasing ethanol and generating silanols. That step doesn’t wait politely for your processing schedule. It speeds up or drags out depending on pH and water content. I’ve watched more than one rookie lose control and get gelling, uneven coverage, or wasted additive. It pays to pre-hydrolyze, giving the silane some water — not too much, or else it gets sticky and useless — and let those silanols appear in a controlled way.
Methacryloxymethyltriethoxysilane shines in systems with glass fiber, mineral fillers, or silica, but only if each particle gets a fair share of attention. Too much silane all at once, and clusters form or things separate. Rushing mixing or skimping on dispersion tools leaves islands of uncovered filler. Good results come by slowly dosing a pre-hydrolyzed silane solution, with steady agitation. I’ve learned not to save time by cranking up the stir bar — that shears fragile particles and tosses dust everywhere. Patience and pulse additions give better coverage with less waste.
There’s no shortcut around properly pre-treating fillers or surfaces. Dust, oils, and old batch residue kill any chance for that methacryloxy group to stick. Every strong composite project I’ve seen gets this right: start with well-cleaned, slightly roughened fillers for silanol groups to latch onto. Surface area, porosity, and actual cleanliness make or break adhesion. Investing time here lifts product performance more than spending extra on additives down the line.
I used to think more silane meant better bonds. Experience (and a few ugly batch failures) changed my mind. At recommended levels — often less than 2 percent based on filler weight — methacryloxymethyltriethoxysilane delivers its best. Extra doesn’t improve things; instead, you start to undermine resin flow, increase viscosity, or spark unwanted side reactions during curing. It matters to respect these limits, measure carefully, and keep records.
This compound isn’t benign. It releases ethanol during hydrolysis, and inhaling vapors or skin contact can bite. Good ventilation, tight gloves, and face shields protect people in the lab and on the plant floor. Collect hydrolyzed waste and spent solvent thoughtfully, and never let it slosh down a drain. Regulations tighten year by year; cutting corners always backfires in the long run.
Many in industry see silane additives as old news, but materials continue to change and regulatory focus only grows sharper. What worked a decade ago might not pass the tests today. Each new resin or filler needs a real-world evaluation. I’ve seen steady improvements come from spending the time on small-batch trials, testing mechanical properties, and gathering feedback from folks using the product outside the lab. Methacryloxymethyltriethoxysilane doesn’t hand out great adhesion for free, but respecting its quirks can shore up manufacturing, reduce customer complaints, and save plenty of headaches down the road.
| Names | |
| Preferred IUPAC name | [ (Triethoxysilyl)methyl 2-methylprop-2-enoate ] |
| Other names |
3-(Triethoxysilyl)methoxypropyl methacrylate 3-(Methacryloyloxymethoxy)propyltriethoxysilane Silane, methacryloxymethyltriethoxy- Triethoxy(3-methacryloxymethoxypropyl)silane |
| Pronunciation | /ˌmeθ.ə.krɪ.lɒk.si.oʊˈmɛθ.əl.traɪˌɛθ.ɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 2530-85-0 |
| Beilstein Reference | 1462205 |
| ChEBI | CHEBI:87134 |
| ChEMBL | CHEMBL1858752 |
| ChemSpider | 167368 |
| DrugBank | DB14055 |
| ECHA InfoCard | 19b991e4-2f4a-4b25-86b4-0c48065c01ef |
| EC Number | 213-934-0 |
| Gmelin Reference | 59734 |
| KEGG | C19206 |
| MeSH | D017209 |
| PubChem CID | 11688579 |
| RTECS number | UB2975000 |
| UNII | VF2F878T0T |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID4036798 |
| Properties | |
| Chemical formula | C11H22O5Si |
| Molar mass | 292.41 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Characteristic |
| Density | 1.045 g/cm3 |
| Solubility in water | Insoluble |
| log P | 0.9 |
| Vapor pressure | <0.1 hPa (20 °C) |
| Acidity (pKa) | 13.2 |
| Basicity (pKb) | 13.5 |
| Magnetic susceptibility (χ) | -7.45×10^-6 cm³/mol |
| Refractive index (nD) | 1.430 |
| Viscosity | 2.5 mPa·s |
| Dipole moment | 3.88 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 245.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3405.8 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H315, H319, H317 |
| Precautionary statements | P280-P261-P305+P351+P338-P337+P313 |
| NFPA 704 (fire diamond) | 1-2-2-Health-1-Flammability-2-Instability-2 |
| Flash point | Flash point: 110 °C |
| Autoignition temperature | 260 °C |
| Explosive limits | Explosive limits: 1.8–11.5% |
| Lethal dose or concentration | LD50 Oral Rat 8025 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat Oral >2000 mg/kg |
| NIOSH | XN1225000 |
| PEL (Permissible) | Not established |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Methacryloxypropyltrimethoxysilane Methacryloxypropyltriethoxysilane Methacryloxymethyltrimethoxysilane Vinyltriethoxysilane 3-Glycidyloxypropyltrimethoxysilane |