
Methoxytrimethylsilane didn't appear in textbooks overnight. In the decades following World War II, organosilicon chemistry caught the eye of researchers chasing more durable polymers and advanced materials. Silylation agents like methoxytrimethylsilane rose to prominence because they could modify organic compounds in ways traditional organic chemicals struggled to match. Chemists adopting newer analytical techniques started seeing the advantage of protecting fragile parts of molecules, which drove the design of reagents like methoxytrimethylsilane. Silicon chemistry has often played second fiddle to carbon, but industrial needs gradually carved out a growing list of roles for these compounds, especially across pharmaceuticals, coatings, and the burgeoning field of semiconductor fabrication.
Methoxytrimethylsilane stands out for its dual personality: part protective group for organic synthesis, part reactive partner in advanced materials. Companies tend to supply it as a clear, colorless liquid with a faint, sweet aroma that hints at its flammable nature. Most bottles arrive sealed under inert gas thanks to its moisture sensitivity. Bulk users—chemical manufacturers, researchers, specialty labs—rely on reliable supply chains because keeping a constant stock of this compound ensures smooth workflows in synthesis and manufacturing lines where delays cost both time and money.
With a molecular formula of C4H12OSi and a molecular weight just below 104 grams per mole, methoxytrimethylsilane is easy to handle compared to bigger, more complex organosilanes. It boils at just under 57°C, which makes it volatile even at room temperature—always a signal to keep the bottle shut tight and avoid any flame nearby. Its refractive index and density fall within ranges common for light organosilicon compounds. The low viscosity encourages quick mixing, but its high vapor pressure puts pressure on lab and plant managers to ensure good ventilation. Methoxytrimethylsilane reacts easily with water and alcohols, so any careless storage or leaking lid leaves users cleaning up hazardous silanol byproducts instead of pushing out the next batch.
Producers stamp every bottle of methoxytrimethylsilane with rigorous labeling that outlines batch numbers, purity, moisture content, packaging date, and safety data. Purity standards usually top out at 98% or higher for most applications—synthetic chemistry generally doesn’t tolerate sloppiness, and minor impurities can wreck a carefully laid out experiment. The safety data must be close at hand, with pictograms signaling its flammability and the risk of respiratory and eye irritation on contact. Commercial containers often arrive in steel or lined drums for industrial users, while laboratories stick with sealed glass ampoules to cut down contamination and evaporation.
Manufacturing methoxytrimethylsilane taps into the reactivity of chlorotrimethylsilane with sodium methoxide or methanol, driving off the sodium chloride as a byproduct—practical, cost-effective, and reliable over long production runs. This method sits at the heart of large-scale synthesis, where side-products are easily filtered out and the final liquid is distilled under reduced pressure. Experienced chemists know that scaling up these reactions involves managing exothermic behavior, ensuring no air or water sneaks into the reactor, and sticking to process safety rules that keep everyone in one piece by the end of the shift.
Methoxytrimethylsilane walks onto the stage as a strong silylating agent. In the lab, it reacts quickly with alcohols, amines, acids, and water—a single misstep leads to cloudiness from silanol formation that no researcher wants to see. Modern organic synthesis leans heavily on this compound for protecting sensitive alcohol groups, especially when building up complex molecules for pharmaceuticals. Sometimes, it gets used to alter surfaces on silica gel or glass, turning hydrophilic materials into hydrophobic tools for chromatography or microfabrication. Sharp-minded researchers dig deep into the reactivity patterns, discovering ways to swap out the methoxy group to tune reactivity and stability in next-generation materials and catalysts.
Anyone picking up a catalog will notice methoxytrimethylsilane wears many names—Trimethylmethoxysilane, Methoxytri(methyl)silane, or simply TMS-OMe. These synonyms confuse newcomers, but industry veterans know it’s the same molecule under different trade dress. Catalog numbers can differ by manufacturer, with Sigma-Aldrich, Alfa Aesar, and TCI Chemicals all offering versions for different markets. Staying alert to these naming conventions cuts down costly procurement mistakes and guards against receiving lower-grade substitutes.
Methoxytrimethylsilane commands the same respect you’d give to any flammable, low-boiling solvent. Safe handling starts with gloves, goggles, lab coats, and fume hoods—cutting corners opens the door to splashes and dangerous vapors that can knock out even seasoned chemists. The substance irritates the skin, eyes, and respiratory tract, so first responders keep neutralizing agents and plenty of water on standby near workspaces. Storage guidelines state the substance belongs away from acids, oxidizers, and sources of ignition, with secondary containment warehousing any bulk drums. Industrial sites often employ gas detection and remote ventilation, preventing unnoticed leaks from reaching explosive limits. Training programs stress exceptional vigilance when transferring from drums, and emergency response plans factor in both cleanup and fire suppression.
Methoxytrimethylsilane turns up across organic synthesis labs, semiconductor fabs, and even specialty coating production lines. In the lab, it earns praise for protecting alcohol groups in multi-step pharmaceutical syntheses. Silicon wafer manufacturers value its ability to deposit hydrophobic layers that guard sensitive electronics from moisture. Researchers tuning chromatography columns modify silica surfaces with this silane to sharpen the separation of target molecules, biting down on run times and improving reproducibility. Outside the lab, paint and coating engineers harness its reactivity to impart water resistance and extend the lifespans of industrial finishes. Its unique chemistry helps scientists build and modify everything from medical diagnostics to architectural surfaces that shrug off rain and grime.
Methoxytrimethylsilane attracts theorists and practical-minded chemists alike who keep pushing its boundaries. Years ago, the main use revolved around protecting functional groups or preparing specialized chromatography phases, but creativity knows no bounds. Modern research focuses on designing smarter materials: hybrid catalysts, surface coatings that repel pathogens, and even platforms for drug delivery. R&D programs in global conglomerates and start-ups pour resources into understanding how minor tweaks in the molecule or reaction conditions translate into big leaps in product performance. As demand climbs for greener, more sustainable processes, company labs explore bio-based feedstocks and energy-saving synthesis lines for this silane.
While methoxytrimethylsilane tends to evaporate quickly, its vapor causes headaches, dizziness, or worse if left unchecked in a closed lab. Toxicity studies show irritation at relatively low concentrations, warning against working in poorly ventilated spaces. Eye and skin exposure calls for thorough rinsing, and chronic handling with insufficient protection risks longer-term respiratory problems. Animal models flag possible risks with high doses, prompting industry and regulatory agencies to push for tighter controls and detailed exposure assessments. Environmental studies dig deeper, revealing that in open air, this compound breaks down quickly, but spilled quantities can contaminate groundwater unless intercepted by secondary containment. Researchers at regulatory bodies and safety watchdogs continue tracking case studies to refine workplace guidelines.
Looking ahead, methoxytrimethylsilane has both looming challenges and ripe opportunities parked on its horizon. As the world leans on advanced materials for things like flexible electronics, wear-resistant surfaces, and smart drug delivery, chemists chasing new frontiers are itching to unlock new uses for silyl group chemistry. Environmental pressure will drive producers to seek cleaner preparative routes, while regulatory scrutiny will tighten rules on handling, usage, and waste treatment. Companies that jump into process innovation—greener chemistries, safer process scale-up, smart packaging—stand to dominate markets that reward reliability and sustainability. I’ve seen labs pivot toward catalysts that demand creative surface treatments, or startups racing to patent next-gen coatings. Methoxytrimethylsilane won’t solve these problems on its own, but few compounds manage to link classic synthetic methodology to cutting-edge applications quite so neatly.
Someone who’s spent a lot of time around a chemistry lab or production plant knows there are certain chemicals that quietly keep a lot of industries humming. Methoxytrimethylsilane ranks high on that list. On the surface, it sounds like just another tongue-twister in a lineup of hard-to-pronounce compounds, but its role in making other things work better is pretty fascinating.
Chemists work with a lot of delicate materials. Processes like organic synthesis, especially in pharmaceuticals and materials science, need tight control over how molecules interact. Methoxytrimethylsilane steps in as what’s called a "silylating agent": it temporarily attaches itself to some sensitive parts of a molecule—like alcohols or amines—so those pieces stop reacting with other stuff during a reaction.
For example, my time in a research lab made me keenly aware of how annoying it is when a protecting group refuses to come off after you’re finished with a step. Methoxytrimethylsilane doesn’t make a fuss. It forms the trimethylsilyl ether quickly and, when you’re ready, lets go without wrecking the rest of the molecule. That saves hours troubleshooting reactions, and it reduces wasted materials.
Outside the lab, methoxytrimethylsilane proves useful for tweaking surface properties in everything from glassware to semiconductors. Glass surfaces, for example, pick up water easily. That creates headaches in electronics or optics. By treating glass with methoxytrimethylsilane, water doesn’t stick and the glass becomes hydrophobic.
I’ve seen coatings like these put to work in electronics clean rooms, where a single fingerprint ruins a batch. In paint and coatings, this molecule keeps surfaces slick and resistant to dust, stains, and even markers. It’s a modest way to cut down on cleaning and repair.
Silicones go into everything from medical devices to cookware. Purity and consistency matter. Methoxytrimethylsilane helps here as a component in making silicone resins and oils. It reacts neatly and helps string together the silicone chains, letting manufacturers control properties like viscosity and flexibility.
You may not see the connection right away, but every time a medical implant comes out without leaching odd chemicals, or a frying pan wipes clean without flaking, it reflects careful chemistry. Chemicals like this underpin that reliability.
Working with methoxytrimethylsilane, like lots of small-molecule organosilanes, asks for respect. It’s flammable and vapors catch easily. In the lab, gloves and eye protection aren’t optional. Proper ventilation means the difference between a safe reaction and a very bad day. Companies must invest in solid training so no one learns the hard way.
It’s tempting to overlook compounds with names like methoxytrimethylsilane, but industrial chemistry relies on building blocks that do their job quietly and efficiently. Demand keeps growing as pharmaceuticals, electronics, and coatings call for smarter, more reliable components. Regulators and manufacturers should keep an eye on both sourcing and safety, remembering that these pieces—while invisible to most—set the stage for much bigger things.
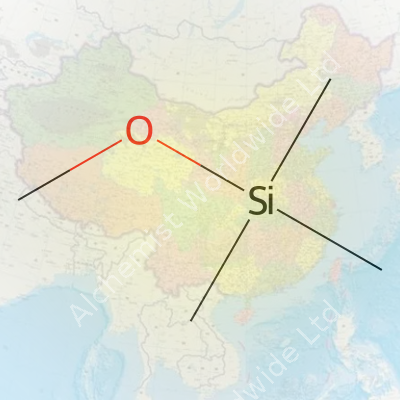
Methoxytrimethylsilane—known to those in the lab as Me3SiOCH3—carries the chemical formula C4H12OSi. Seeing those letters and numbers brings back memories of balancing equations in organic chemistry, where each symbol represents much more than just a component in a reaction. This compound plays a consistent role in both research and production settings, so knowing the structure and properties makes a difference beyond any textbook answer sheet.
The backbone of methoxytrimethylsilane sits at a connection between silicon and oxygen—two elements that don’t always find themselves bonding in quite this arrangement. Three methyl groups attach to the silicon atom, with the fourth carbon and extra hydrogen and oxygen making up the methoxy part. Chemists rely on the precise nature of this formula during synthesis and purification steps. Incorrect formulas in a lab notebook could ruin a costly experiment or throw off a large-scale process in industry.
Methoxytrimethylsilane finds its value in protecting hydroxyl groups. Synthetic organic chemists use it while building complex molecules, blocking parts of a molecule from reacting. The compound makes an appearance in creating pharmaceuticals, electronic materials, and specialty polymers. Protection and deprotection steps often add a level of both challenge and creativity to chemistry, and this molecule’s formula enables that flexibility. I remember a postdoctoral researcher explaining how skipping a protection step with the wrong silyl ether led to a failed synthesis after weeks of work. Details like C4H12OSi matter when stakes climb.
Reliable information about methoxytrimethylsilane’s formula feeds directly into safe storage and use. In a university lab, I once encountered confusion when a bottle showed only a trade name. Colleagues puzzled over whether we could use it as a methylation agent. Checking the formula on a trusted database cleared up the confusion and prevented a hazardous misapplication. Silanes can emit flammable vapors, and proper labeling along with an accurate understanding of the molecule minimizes exposure risk.
Students and professionals often stumble through the maze of ingredient names—trade, systematic, or slang. Consistently referencing the actual chemical formula builds clarity. Professors encourage learning to interpret not just names but also the structure and implications of a compound. Tools like spectral databases and interactive molecule viewers help turn abstract formulas into concrete knowledge. In lab meetings, visualizing a structure based on C4H12OSi clarifies why methoxytrimethylsilane reacts the way it does.
Every chemist faces a tidal wave of information. The skills to track down reliable data sources grow with experience. I keep digital links to original data sheets, spectral libraries, and standard references close at hand. That habit saves time and builds a safety net against simple but costly errors—like misidentifying a bottle or trusting a supplier’s unlabeled sample. It’s a habit born out of lessons learned in real labs and not just late-night study sessions.
Knowledge of chemical structures like C4H12OSi forms a piece of the bigger puzzle in advancing materials, medicines, and processes. Someone new in the field soon discovers why accuracy in naming and formula makes or breaks both discovery and safe practice. The basic formula helps guarantee that progress continues without unnecessary setbacks.
I’ve worked plenty of years in labs, and I’ve seen firsthand how overlooking the basics with chemicals like methoxytrimethylsilane leads to more than an academic headache. It smells a bit like nail polish remover, and because of that, some folks downplay it. But here’s what sticks with me: it isn’t just a volatile liquid, it’s a highly flammable one that doesn’t need much encouragement to catch fire. Keeping this compound in check means more than finding a shelf and walking away.
This isn’t something you toss into any old cabinet. I always make sure it sits in a cool place with real ventilation and I never forget the flammables cabinet. I once saw a bottle left near a window during summer, which sounds harmless until you see the heat haze on glass and realize that even moderate sunlight can send temperatures climbing fast. A fire at that temperature spreads before you can grab a spill kit.
Tightly sealed containers are a must. Silanes like this draw in moisture from air. A cracked or loose cap isn’t just bad lab etiquette—it starts a slow, unwanted reaction with air or water. That leads to fumes, pressure buildup, or even explosive situations. Silane’s not forgiving here.
I’ve helped train new hires, and one lesson never changes: always work in a fume hood. People talk up gloves and goggles, but many forget consistent airflow matters even more. Vapors can catch in your throat before you notice them. I stick to nitrile gloves and eye protection because this liquid’s keen to irritate eyes and skin. The point isn’t paranoia—it’s protecting your own hands and lungs long-term.
Sometimes colleagues ask if it’s overkill to prep spill kits, sand, and extinguishers before measuring out even small volumes. Once, someone tried to mop up a spill with paper towels. The reaction between methoxytrimethylsilane and water in the towels nearly caused a panic. Now, I always insist on inert absorbent and always wear a lab coat. Fire extinguishers, especially carbon dioxide or dry chemical type, are better than water, which can just spread things out and make it worse.
The statistics don’t lie. The U.S. Chemical Safety Board reported dozens of accidents over the last decade that trace back to poor storage or handling of reactive silicones. Many labs and facilities already have protocols in place, but shortcuts often creep in over time. Sometimes it takes a small accident to remind folks what’s at stake.
Updating training for everyone, not just the new folks, keeps the dangers fresh in mind. Regular audits, clear signage in storage rooms, and easy access to safety data sheets help too. If you’re short on flammable storage, don’t just make do—raise the alarm and get the right gear. Management sometimes forgets that safe storage keeps both people and money intact. You only need a single misstep with a bottle of this stuff to lose the trust of those relying on the safety net you promised them.
Methoxytrimethylsilane deserves more than routine caution. My experience tells me that the habits we build around safe chemical storage and use aren't just paperwork—they make sure everyone gets home at the end of the day, every day.
Chemicals crop up in daily life more often than most folks realize. From cleaners to car parts, there's usually something in the fine print that looks straight out of a science lab textbook. Methoxytrimethylsilane is one such name. Used in labs and factories for making other chemicals or modifying surfaces, it doesn’t have the recognition of household items, yet it deserves as much respect during handling.
Information from reliable sources like chemical producers and regulatory bodies matter here. Methoxytrimethylsilane has flammable properties and reacts with moisture to produce methanol and other byproducts. Methanol causes trouble — touching or breathing in enough can make you dizzy, nauseous, or worse. The chemical itself gives off strong fumes that can irritate the nose, lungs, or skin if it slips past basic protection. A splash in the eye stings and can leave lasting damage without a quick rinse.
Factories and research sites use dedicated equipment like goggles, gloves, and fume hoods for a reason. It’s not about being overly cautious — it’s about preventing some real pain, lost work time, and long-term health issues. The CDC, OSHA, and Europe’s REACH program all keep tabs on chemicals like this. Their advice isn’t just paperwork. It’s born from investigations into accidents and near-misses.
Methoxytrimethylsilane breaks down quickly in the environment once exposed to water or air. That means it doesn’t build up in soil or waterways. Still, in enclosed spaces or in an accidental spill, the risk gets real. The fumes reach airways, and reactions can develop fast. Large doses of methanol, its breakdown product, may damage eyesight or harm the nervous system. Reports from poison control centers document that methanol ingestion, even in small amounts, can have devastating impacts, sometimes fatal, and it’s tough to treat after a delay.
I’ve seen close calls in labs where someone forgot to wear gloves or skipped the fume hood because they were “just cleaning up a spill.” That can lead to red eyes, headaches, and in serious situations, hospital trips. Employers hammer home basic chemical safety because the consequences are real. Even if industry guidelines feel like overkill, there’s hard-won wisdom in those warnings.
Information and simple protection make the biggest difference. For everyday workers or students, that means goggles, gloves made for chemicals, and an exhaust fan or fume hood switched on before starting the job are non-negotiable. Knowing where the eyewash station and safety shower are saves precious seconds in emergencies. Supervisors encourage people to speak up if something doesn’t feel safe, since even a single shortcut could mean an accident.
For risk outside workplaces, the chance of significant methoxytrimethylsilane exposure stays low unless you spend a lot of time around industrial processes or chemical prep areas. Product safety sheets and hazard labels hold answers. If curious about a chemical, look them up and listen to public health guidance. I’ve built trust in the advice of chemists, emergency responders, and the folk who run poison control — their knowledge and experience outweigh gut feelings every time.
Manufacturers can push for safer packaging and more readable warning labels. Training new workers well and keeping up on chemical handling rules protects both people and the business. For anyone handling these chemicals at home or onsite, using the right gear and cleaning up spills fast prevents a bad day from turning into a disaster. Spreading honest, practical advice does more good than vague promises of safety.
Chemists often expect Methoxytrimethylsilane to land at a purity range near 98%-99%. This expectation didn’t come out of nowhere. The world of silicon-based reagents leans heavily on dependable quality. If impurities sneak in, they can jam up a reaction or send yields in the wrong direction. Labs and manufacturers pay extra for the 99% level most times. If a process absolutely can’t handle surprises, some specialty suppliers push that number higher—think 99.5% or even better for critical applications.
Quality control teams run the numbers after a batch comes out the other side of production. Gas chromatography quickly picks up on anything that shouldn’t be there. Some facilities go further by checking for water, since even low-moisture levels can ruin sensitive syntheses. Additives rarely find a place in high-purity grades. The result is a product that doesn’t just meet paperwork standards, but keeps synthetic routes predictable.
A researcher or process chemist often finds Methoxytrimethylsilane packed in 100 mL glass bottles. This size works for bench-scale experiments—enough for a few dozen reactions without running up storage worries. Some universities and small research outfits rely almost exclusively on this standard bottle size. Zero waste, convenient to handle.
Bump the scale up, and one-liter bottles become more common. Pharmaceutical plants, custom manufacturers, and production chemists usually ask for bigger volumes. Five-liter metal cans, or specialty coated drums, appear in settings where bulk orders actually make sense. When the project can eat through kilograms fast, only drum packaging slows down procurement bottlenecks. Engineers have to consider regulations for flammables and air-reactive materials, so packaging quality and seals receive careful scrutiny.
Methoxytrimethylsilane carries a set of hazards. The compound reacts with air and water, sometimes with enough force to threaten glassware or spark an unexpected cleanup session. I’ve seen a well-sealed cap give way on a humid day, and nobody wants to step into a lab during that surprise. That’s why most suppliers stick to bottles with solid Teflon-lined or special polymer seals. Some vendors double-bag glassware in aluminum sleeves for shipping, especially when temperatures swing.
Companies handling production quantities often ask vendors for certificates of analysis before shelling out for a pallet. Any sign of water in the sample means the supplier’s storage or seals lagged behind standards. Strict labeling, up-to-date hazard pictograms, and clear lot information make it easier for a lab manager to track inventory and recall materials if needed.
Meeting purity standards feels fairly straightforward in North America, Europe, and Japan. Labs in other regions still face gray-market suppliers, bottling inconsistencies, and storage issues. Sometimes a cheap batch arrives at 95% purity—fine for cleaning glassware, less so for catalysis. To fix this, bigger companies partner with distributors that invest in proper warehousing and handling protocols.
Certification efforts backed by ISO or REACH help keep both big and small buyers away from cut corners. For researchers operating on limited budgets, group purchasing can leverage trustworthy suppliers without the risk of mystery contaminants wrecking months of work.
Good recordkeeping and supplier audits help drive purity levels higher year by year. Researchers new to Methoxytrimethylsilane should treat low-purity lots with skepticism, even if the price dips. Keep close relationships with technical reps, review safety sheets for each batch, and push suppliers for packaging that stands up to both transport and long storage. If the right purity and packaging match the need, a lab runs smoother—and safety headaches shrink, too.
| Names | |
| Preferred IUPAC name | trimethoxy(methyl)silane |
| Other names |
Trimethylmethoxysilane Methoxy(trimethyl)silane Me3SiOCH3 TMMS |
| Pronunciation | /mɛˌθɒksiˌtraɪˌmɛθɪlˈsaɪleɪn/ |
| Identifiers | |
| CAS Number | 2857-97-8 |
| 3D model (JSmol) | `3Dmol.js?model=CCO[Si](C)(C)C` |
| Beilstein Reference | 1465067 |
| ChEBI | CHEBI:75442 |
| ChEMBL | CHEMBL156443 |
| ChemSpider | 84111 |
| DrugBank | DB14171 |
| ECHA InfoCard | 07afc1b0-6b34-43bb-b6d8-3e88de462fd0 |
| EC Number | 200-933-1 |
| Gmelin Reference | 85877 |
| KEGG | C19322 |
| MeSH | D008756 |
| PubChem CID | 66131 |
| RTECS number | KN0175000 |
| UNII | VT773FJ11Z |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID6051599 |
| Properties | |
| Chemical formula | C4H12OSi |
| Molar mass | 106.22 g/mol |
| Appearance | Colorless liquid |
| Odor | sweet |
| Density | 0.765 g/mL at 25 °C |
| Solubility in water | Insoluble |
| log P | 0.8 |
| Vapor pressure | 13.5 kPa (20 °C) |
| Acidity (pKa) | 18.2 |
| Basicity (pKb) | 7.6 |
| Magnetic susceptibility (χ) | -63.0e-6 cm³/mol |
| Refractive index (nD) | 1.369 |
| Viscosity | 0.46 mPa·s (25 °C) |
| Dipole moment | 3.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 288.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -240 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1946.6 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | Precautionary statements: "P210, P261, P280, P304+P340, P312, P370+P378 |
| NFPA 704 (fire diamond) | 1-4-0-✕ |
| Flash point | -1 °C |
| Autoignition temperature | 287 °C |
| Explosive limits | Lower: 1.3% Upper: 16% |
| Lethal dose or concentration | LD50 (oral, rat): 2100 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 21000 mg/kg |
| NIOSH | B017 |
| REL (Recommended) | REL: 50 ppm |
| IDLH (Immediate danger) | IDLH: 1,500 ppm |
| Related compounds | |
| Related compounds |
Chlorotrimethylsilane Trimethylsilyl trifluoromethanesulfonate Trimethylsilyl iodide |