
Methylphenyldiethoxysilane, a compound bearing the formula C11H18O2Si, began turning heads among chemists back in the postwar years, during the early surge of organosilicon chemistry. Researchers explored avenues far beyond glass and ceramics — they valued silanes for their unique ability to link organic and inorganic worlds. As polymer science branched out in the 1960s, interest in trialkoxysilanes and their analogs, such as methylphenyldiethoxysilane, grew rapidly. Chemical industry players identified its role in the synthesis of functionalized silicones and high-performance materials. This compound's story is stitched into the broader narrative of specialty silanes fueling industrial and materials innovation, especially in the electronics and coatings fields in East Asia, Europe, and North America.
Methylphenyldiethoxysilane sits in an interesting niche. It's neither the most popular silane for basic crosslinking nor a rare laboratory oddity. Chemists use it as a key precursor for tailored silicone polymers and surface treatment agents. Its structure — a methyl group and a phenyl group bound to silicon, flanked by two ethoxy groups — opens doors for both reactivity and stability. On the supply side, specialty chemical suppliers keep it in their stock catalogs for research and industrial buyers, especially those in coatings, adhesives, and advanced sealants. Laboratories value its predictable behavior and the straightforward hydrolysis posted by the ethoxy groups, making it a smart base for subsequent modifications.
At room temperature, methylphenyldiethoxysilane appears as a colorless to pale yellow liquid. It often emits a faint aromatic smell, a clue to its phenyl content. Density usually falls in the 0.96–1.02 g/cm³ range, with a boiling point near 260°C. It does not mix readily with water but dissolves in most common organic solvents like toluene, ether, and alcohols. Chemically, the compound presents a combination of hydrolyzable ethoxy groups and aromatic stability. It resists rapid oxidation and mild acids, yet water in excess will slowly hydrolyze the ethoxy units to form silanols, which then condense to siloxanes. This versatility supports both storage stability and controlled use in synthesis.
Companies bottle and ship methylphenyldiethoxysilane with clear specifications. Purity levels typically exceed 98%, with residual moisture and volatile organic acids tightly controlled below 0.05%. Labels carry hazard symbols—most standard for flammable substances and those presenting moderate skin or eye irritation risk. Material safety data sheets spell out handling protocols, disposal guidelines, and transport codes. For traceability and regulatory compliance, batch numbers and analysis certificates accompany commercial shipments. Regulatory harmonization means packages align with GHS labeling, offering buyers consistent safety and identification information regardless of their country.
Industrial production begins with phenylmethylchlorosilane as a key raw material, common from the direct process reacting methylchlorosilane, phenylchlorosilane, and copper catalyst. Ethanol or sodium ethoxide acts as the ethoxylating agent, coaxing out the two desired ethoxy groups through a substitution reaction that releases hydrogen chloride or sodium chloride as the byproduct. The final product distills off to remove low-boiling impurities. The detailed process often involves moisture-free conditions and inert atmospheres since hydrolysis can ruin yields quickly. Many labs fine-tune reaction times, temperature ramps, and solvent choices based on downstream application or purity standards.
Chemists value the ethoxy arms of methylphenyldiethoxysilane most. These groups hydrolyze under acidic or basic catalysis, generating silanol intermediates. These silanols condense with other silanols or with hydroxylated surfaces, knitting robust silicon-oxygen bonds that anchor the silane to glass, ceramic, or organic polymer backbones. The methyl and phenyl substitutions on the silicon atom also shape the final material's hydrophobicity and thermal properties, allowing scientists to control polymer flexibility, optical characteristics, or chemical resistance. Some research teams graft additional organic groups onto the aromatic ring, aiming for specialty polymers or functional coatings with anti-fouling or biomedical applications.
A walk through different supplier catalogs reveals plenty of alternate names. Chemists and purchasing agents may know it as methylphenyldiethoxysilane, but it turns up as phenylmethyldiethoxysilane, (diethoxy(methyl)(phenyl)silane), or by various registry numbers like CAS 14783-49-4. Specialty brands assign their proprietary codes if they blend or distill their own version. Manufacturers in Europe and Asia translate or abbreviate designations, but chemical databases maintain a web of cross-references to minimize confusion. Accurate names in technical documentation prevent shipping errors and regulatory headaches.
Managing methylphenyldiethoxysilane demands solid workplace protocols. The liquid catches fire readily, so it belongs nowhere near open flames, sparks, or oxidizers. Spills on the skin usually cause irritation within minutes, especially on sensitive skin or broken barriers; eye contact needs rapid flushing and likely medical follow-up. Operators wear goggles, gloves, solvent-resistant aprons, and keep spill kits ready. Well-ventilated hoods limit inhalation risk. Standard operating procedures focus on quickly sealing drips, using only approved transfer lines and pumps, and logging all material usage. Waste solutions require collection in labeled containers, avoiding release into drains or unmarked holding tanks.
Methylphenyldiethoxysilane lands in a surprising number of modern technologies. Silicone rubber manufacturers rely on it for building blocks, especially where a subtle mix of flexibility and thermal stability matters, like in automotive or aerospace seals. Glass and ceramic producers use its functional groups for surface modification, making their substrates more compatible with organic coatings or adhesives. Electronics fabricators harness its properties in encapsulants, insulating gels, and even as minor additives in advanced photoresists. Researchers in organic electronics and biomedicine push its chemistry further, attaching tailored molecules to the aromatic ring or swapping ethoxy groups for more exotic ligands.
Laboratories working with methylphenyldiethoxysilane focus on new surface treatments, adaptive polymers, and crosslinkers for advanced materials. Scholars experiment with the compound as a precursor in sol-gel chemistry or as a platform for grafting antimicrobial or sensing groups onto consumer product surfaces. Some projects target improved dielectric polymers for flexible electronics, seeking better trade-offs between insulation, elasticity, and lifetime under repeated flexing. Every year, patent literature grows thicker with new compositions and devices built on silane-modified matrices. Collaboration between industrial and academic teams fosters deeper understanding and broader commercial pathways, fueling demand for pure, consistent supplies.
Any chemical with active ethoxy silane groups and an aromatic core deserves scrutiny. Studies in occupational health highlight the irritant effects of methylphenyldiethoxysilane — repeated or prolonged exposure can inflame skin, eyes, and mucous membranes. The compound’s volatility is modest, yet accidental inhalation or spillage creates risk. Animal model data reveal low acute toxicity, but structural similarity to known hazardous silanes prompts ongoing review. Green chemistry advocates campaign for even less toxic alternatives and push for closed-cycle synthesis facilities. Effective workplace ventilation, proper personal protective equipment, and incident reporting reduce risk, but safety training and strict inventory controls matter most.
Industries chasing ever-thinner electronics, smarter coatings, and greener building materials keep methylphenyldiethoxysilane on their lists. As climate policies spur demand for tougher, longer-lived polymers or functional interfaces, its role may grow in both classic and emerging markets. Chemists see room for designing new analogs—swapping out aryl or alkyl groups, tweaking reactivity—to tailor final properties closely to target uses. Sustainability concerns push producers and buyers to manage waste, recycle solvents, and seek out lower-impact alternatives while meeting high technical performance standards. The conversation between regulatory agencies, industry leaders, and research labs shapes the future for all specialty silanes, and methylphenyldiethoxysilane's journey will mirror those trends.
Methylphenyldiethoxysilane doesn’t exactly roll off the tongue, but its value stretches through several parts of the chemical industry. Once you’ve spent a few years around coatings, adhesives, or even specialty plastics, the name pops up once in a while. The compound itself sits among organosilicon chemicals. What separates it from the crowd is its blend of organic and inorganic features. The “methyl” and “phenyl” parts bring organic character, giving flexibility and resilience, while the “diethoxysilane” segment helps it form strong chemical links with silicates and glass surfaces.
From personal experience, the first time I learned about methylphenyldiethoxysilane came up during a discussion on surface treatment for glass fibers. In jobs like that, tough conditions push regular coatings to the limit. An ordinary connector or adhesive can peel away from glass, but this silane offers something better—a way to bridge glass and polymer, turning a slippery surface into something adhesives actually grip. Paint manufacturers value it for that reason; it helps paint stick longer and resist weather damage.
Electronics companies also take advantage of it. The growing need for electronic circuits in smaller, stronger packages means the old ways don’t cut it. Methylphenyldiethoxysilane plays a role in forming thin, durable insulating films on chips or circuit boards. Instead of letting moisture sneak in and ruin a silicone seal, this silane adds an extra layer of resistance. Some top-end LED makers swear by it because it helps keep electrical parts dry.
There’s real impact here, and it isn’t just about technical performance. Paints and coatings built with methylphenyldiethoxysilane last longer, so buildings need to be painted less often. Higher durability saves on maintenance costs and cuts waste. In electronics, the risk of failure from moisture or heat drops, which means fewer devices end up in landfills after a short life. The push for greener industries can’t ignore compounds that lead to longer-lasting goods.
Getting hands on pure methylphenyldiethoxysilane isn’t easy. Reliable suppliers matter, since impurities can ruin the whole batch. It’s not a DIY chemical—handling it without proper training exposes workers to risks, including vapor inhalation or burns. Facilities using the compound need solid ventilation and protective gear policies. Mistakes can lead to bigger problems than just wasted product. Trust plays a part in the supply chain; working with companies that follow safety and environmental guidelines matters a great deal here.
Experts encourage anyone using methylphenyldiethoxysilane to set up waste capture or recycling programs. Leftover material shouldn’t hit the regular trash. Instead, teams can collect and reprocess it, or send it through approved chemical waste channels. By switching to closed mixing systems, companies lower the chance of spills or fumes in the air. Proper training pairs with the right equipment to keep people safe and cut environmental risks. These moves cost a bit more upfront, but savings stack up over time—less product lost, fewer accidental exposures, fewer environmental fines.
Innovation keeps moving. Chemists and engineers continue experimenting with methylphenyldiethoxysilane in emerging fields like flexible electronics and advanced composites. As demand grows for more reliable, rugged materials, this compound often leads research to the next stage. My years around labs and manufacturing floors show me that when a chemical brings both performance and durability, it keeps earning its place in new products.
Every chemist I know has a story about an overlooked container—a sticky mess, a whiff of something sharp in the air, maybe even an alarm going off. Chemicals like Methylphenyldiethoxysilane don’t forgive simple mistakes. Silanes can react with moisture to create flammable hydrogen gas, which means a casual approach quickly invites danger. Safety here does way more than check a regulatory box. When you keep this liquid under control, you keep people safe and minimize the odds of lost material, injuries, or costly cleanups.
Storing Methylphenyldiethoxysilane in tightly sealed steel or glass bottles with Teflon caps slows down the risk of leaks or contamination. I’ve seen cheaper caps crack over time, leaking out small amounts of vapor you only notice when you feel that tell-tale tickle in your nose. The container should sit in a cool, dry spot, away from sunlight, since UV rays can break down the compound and push up storage risks. Keep this bottle separate from acids, bases, and most oxidizers. If you share shelves with any compatible solvents, double-check spill trays and secondary containment. I remember one incident where a cracked tray left two liquids mixing under a cabinet—simple measures prevent hours of panic.
Methylphenyldiethoxysilane hates humidity. Even a short exposure will start a slow reaction, generating gas and producing by-products you don’t want released in a crowded prep room. Every transfer should happen with dry glassware. If you work in a particularly damp region, silica gel packets and moisture indicator cards can help. People who skip this step usually find tiny water droplets inside the lid after a few weeks, and wonder why things smell off or pressure builds up. It only takes a single event to drill in the lesson that a dry lab space costs much less than a clean-up.
I like to keep my bench in a well-ventilated spot, or better yet a chemical fume hood. Working with silanes in a closed-off or stuffy area just isn’t worth the risk—headaches and worse can creep up on you fast. Anyone handling bigger volumes should consider local exhaust ventilation to tackle vapors. For spills, never use water. I’ve watched colleagues try to dilute a spill with a quick splash, not realizing that reaction means more hydrogen and heat. Instead, soak up with sand or a commercial spill kit, ventilate, and then clean the area with dry rags.
Nobody relishes the thought of suiting up, but I’ve seen even experienced chemists learn the hard way that gloves, goggles, and a splash-proof coat make a difference. The lightweight, clear compound seems harmless until a drop hits your skin or eyes. Short sleeves and typical cloth gloves can’t hold up. Deep training, beyond just reading the SDS, builds habits over time. An eye wash and safety shower nearby look like overkill—right up until they aren’t.
Limiting how much you keep in the lab lowers risks for everyone. Just-in-time inventory works well when a supplier is reliable. Every bottle dated, every lid checked, and every expired product moved to hazardous waste promptly—these small steps add up to a safer, more productive lab. Records not only cut down on waste, they save lives and money in the long run.
Every chemical tells a story as much as it does a job. For methylphenyldiethoxysilane, its chemical formula reads as C12H20O2Si. That means you have twelve carbon atoms, twenty hydrogens, two oxygens, and a single silicon atom brought together. As for its molecular weight, it lands at 224.37 g/mol.
These numbers might not light up a room during small talk, but they pull big weight in real-world uses. Silicon-based chemicals carry a certain mystique in materials science—tough, versatile, and able to bond with organic and inorganic substances. Knowing the exact formula and weight stands as a starting block. Anyone handling chemicals needs to trust in their numbers; a slip in the formula or even a decimal in weight could mean wasted resources at best, and safety hazards at worst.
In the lab or on the production line, guessing doesn’t fly. Teams trust suppliers to state the exact composition of a chemical, and this isn’t only about formality. It’s about safety, regulatory compliance, and reproducibility. The more information provided about a molecule’s makeup, the easier it is to spot problems before they grow. A chemist reading a spec sheet with C12H20O2Si listed doesn’t need to wonder about contaminants or mislabeling. Public trust grows when companies provide clear, accurate product information, and that extends out into end-user safety.
I’ve worked alongside researchers who prepare dozens of batches in a day. They measure out grams of chemicals on busy benches, double-checking each number. If the molecular weight was off, so would every calculation for dosing and mixture. It isn’t uncommon to see someone pull out a calculator to make sure a tenth of a gram matches exactly what’s needed for an experiment. Over time, mastering these basics allowed our team to spot faulty batches before they reached production—saving money and time.
This chemical, with its methyl and phenyl groups tied to silicon and ethoxy partners, sets the tone for how a compound reacts. Molecular weight drives everything from shipping costs to reaction kinetics. Methylphenyldiethoxysilane may show up as a clear liquid, but if assumptions sneak in about its properties, entire research projects risk falling apart. In industries using silanes for coatings or adhesives, even a slight miscalculation raises the risk of product failure.
One concern: consistent quality. Even with a correct formula and proper labeling, cross-checking batch purity and supplier records can form a habit. Analytical testing, like NMR or mass spectrometry, backs up these numbers. I've seen situations where labs order a new supplier only after seeing strong proof of accuracy and transparency. In a world of global trade, third-party audits and digital records guard against mistakes and fraud.
Education also shapes outcomes. Everyone from a student to an engineer benefits when fundamentals like the chemical formula and molecular weight come baked into training and routine checks. Quality control officers walk the talk every day—keeping everything traceable, verified, and easy to access during surprise inspections.
Building a safer and more trustworthy industry starts here: know what sits in the bottle, lean on clear facts, and keep skill sharp with regular checks. A formula printed with confidence doesn’t just fill a line on a data sheet; it protects people, property, and years of collected know-how.
Methylphenyldiethoxysilane isn’t something most folks run into on an everyday basis. You’ll track it down mostly in labs, factories, or specialty manufacturing rooms where chemists tweak silicones or work up coatings and adhesives. This kind of chemical advances certain plastics and resins, making products tougher and more resistant to heat or wear.
Hazards don’t always jump out just because a name sounds unfamiliar. In my own work in chemical handling, nothing sets off alarms more reliably than chemicals with tricky names and little public data. Methylphenyldiethoxysilane is classified as an organosilane, and these compounds have a way of being flammable, as well as irritating to skin and eyes. According to safety data from trusted chemical manufacturers, the liquid releases vapors that irritate noses, throats, and lungs, something I experienced the hard way early in my career by skimping on a mask.
Breathing in too much vapor doesn’t only lead to coughing or a sore throat; it sometimes gives you headaches or dizziness. If the chemical lands on your hands, redness or itching often follows. Eye contact stings and leaves you blinking for half the day. Drinking it would probably send you to the ER. That happens with many solvents and flammable chemicals, so this isn’t a hazard unique to methylphenyldiethoxysilane.
A question that deserves careful attention asks if repeated exposure could build up and lead to bigger health issues. Many chemicals, including some silanes, can act tough on the liver or nervous system after months or years, especially in jobs lacking solid safety gear. Industry experience and reviews from organizations such as the European Chemicals Agency mention that this compound may cause organ damage with repeated exposure over long stretches.
For jobs that use this silane, gloves rated for solvents and eye shields matter—there’s no skipping protection. Good airflow in a workspace means fewer risks of vapor headaches and lung aches. Workers learn early to read Safety Data Sheets, which clearly state “Keep away from open flames” and “Never inhale dust or vapor.” Spills can happen with any fluid, so kits for containment and neutralization stay close by in busy rooms. Skin or eye contact demands fast rinsing with water, sometimes for at least fifteen minutes.
Some companies switch to silanes with a lower vapor pressure, or seek out coatings technology demanding less hazardous ingredients. From what I’ve seen, consistent safety training goes the furthest—people need to respect these chemicals and fully understand what could go wrong.
Proper labeling and sealed storage help avoid accidental exposure. Air monitors for volatile organic compounds, along with regular health checks for staff, help spot signs of overexposure before they become a real problem. Sharing incident reports between factories and chemical suppliers gives everyone fresh lessons to learn from.
Most consumers won’t bump into methylphenyldiethoxysilane at home, but those behind the scenes deserve workplaces that put easy access to information, strong ventilation, and protective equipment before production deadlines. Every product we use starts with choices like these. Knowing the risks keeps makers, engineers, and their families healthy.
Methylphenyldiethoxysilane may not sound familiar, but it touches plenty of products people rely on. This compound is a common pick for surface modifiers in paints, coatings, and sealants. On its own, glass has weaknesses—thin layers of water like to seep in, and dust grinds away at the sheen. Once this silane coats a surface, it links up with other molecules and forms a tough, water-repelling shield. People in construction notice that treated glass doesn’t fog up as much, and painted walls shed stains and dirt easily. The coating even helps graffiti wash off with less elbow grease.
Silicone-based adhesives often list methylphenyldiethoxysilane in their recipe. Manufacturers pick it for jobs where bonds need to survive wild swings in temperature. In the electronics sector, devices heat up and cool down all the time; adhesive failure would start fires or break circuit boards. This silane forms sturdy chemical bridges between plastic, glass, and metal, blocking out moisture and stopping cracks from forming. Research from the Journal of Adhesion Science and Technology points out how silanes can improve bond strength by up to 50% compared to untreated surfaces.
Phones, laptops, and car sensors meet harsh conditions. Water, oils, and grit can ruin delicate circuits or cause data failures. By using methylphenyldiethoxysilane, electronic makers apply thin films that protect the tinniest wires and chips. Some of the biggest names in automotive electronics use silane finishes because the result shields circuits without making things bulky or heavy. It’s not about making parts last forever, but squeezing out more years before repairs or replacements become necessary.
Inside clinics and hospitals, patient safety ties closely to surfaces that shrug off bacteria and harsh scrubbing. Medical tubing, catheter coatings, and dental tools often bear traces of this silane. Its water-repellent and smooth finish discourages bacteria from gripping onto medical equipment. Cleaning teams also appreciate how these treated surfaces resist wear from disinfectants and repeated washing. According to the American Society for Microbiology, the right coating reduces microbial buildup by over 80% compared to untreated plastics.
Outdoor jackets, upholstery, and airbags benefit from silane chemistry. Once textile producers dip fibers in methylphenyldiethoxysilane, rain and oil both slide right off. People who hike in the rain or work long hours in uniform find their gear stays dry and doesn’t stink up as fast. Even airbags, hidden until one critical moment, rely on surface treatment to keep material strong and smooth years after sitting folded up inside a dashboard.
Widespread use of methylphenyldiethoxysilane comes with responsibilities. Factories need ventilation and proper safety gear since inhaling silane fumes brings health risks. Wastewater from silane processing should pass through environmentally certified treatment systems. Progress in green chemistry now gives more options for recycling treated plastics and cutting toxic byproducts. For companies aiming to use silanes, regular staff training and tough oversight on disposal practices make all the difference for health and safety in the workplace and beyond.
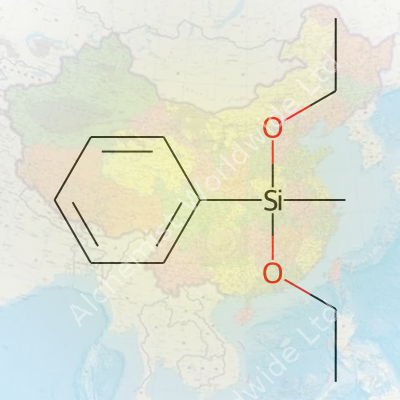
| Names | |
| Preferred IUPAC name | Methoxy-bis(ethoxy)phenylsilane |
| Other names |
Diethoxy(methyl)phenylsilane Phenylmethyldiethoxysilane Diethoxyphenylmethylsilane |
| Pronunciation | /ˌmɛθ.ɪlˌfɛn.iˌdaɪˌɛθ.ɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | [25565-50-2] |
| Beilstein Reference | 1206574 |
| ChEBI | CHEBI:87135 |
| ChEMBL | CHEMBL1432266 |
| ChemSpider | 86719 |
| DrugBank | DB14641 |
| ECHA InfoCard | 18e367f3-61fd-41cf-ad82-3db8aae8b18b |
| EC Number | 205-487-5 |
| Gmelin Reference | Gmelin 390833 |
| KEGG | C19551 |
| MeSH | D017105 |
| PubChem CID | 69482 |
| RTECS number | VV7875000 |
| UNII | T3KG7Q1U8P |
| UN number | 1993 |
| CompTox Dashboard (EPA) | DTXSID4059479 |
| Properties | |
| Chemical formula | C12H20O2Si |
| Molar mass | 256.39 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Aromatic |
| Density | 0.997 g/mL at 25 °C |
| Solubility in water | Insoluble |
| log P | 2.8 |
| Vapor pressure | 0.3 hPa (20 °C) |
| Acidity (pKa) | Acidity (pKa): 16.5 |
| Basicity (pKb) | 13.0 (pKb) |
| Magnetic susceptibility (χ) | -63.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4790 |
| Viscosity | 1 mPa·s |
| Dipole moment | 2.21 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 367.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -356 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| Flash point | 80 °C |
| Autoignition temperature | 430 °C |
| Lethal dose or concentration | LD50 (Oral, Rat): > 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 7.7 mL/kg |
| NIOSH | GV5825000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 ppm |
| Related compounds | |
| Related compounds |
Methylphenyldimethoxysilane Phenyltriethoxysilane Methyltriethoxysilane Phenyltrimethoxysilane Dimethyldiethoxysilane |