
Silicon chemistry has shaped industrial science in practical ways for decades. As new materials emerged in electronics, construction, and coatings, chemists started diving deeper into organosilicon compounds. In the search for improved adhesion, water resistance, and cross-linking abilities, methylpropyldiethoxysilane began to grab attention a few decades back. Demand grew for silanes that didn’t just bond, but offered flexibility for chemical modification—opening doors for hybrid materials. Once researchers figured out how trialkoxysilanes could link organic and inorganic materials, methylpropyldiethoxysilane joined the portfolio for functional silanes sold by companies worldwide. Its significance scaled up with advances in polymer science, especially after the 1970s, as industries searched for new ways to coat, seal, and protect surfaces, and as demand increased for tailored adhesion in high-tech manufacturing.
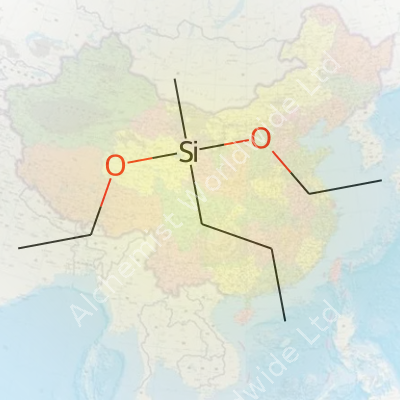
Walk through a facility producing engineered plastics or specialty glass and you’ll find methylpropyldiethoxysilane among the raw materials. This compound brings together a methyl and a propyl group bonded to silicon, plus two ethoxy groups that allow it to react with water and other substrates. It fits right into processes needing a balance between hydrophobic properties and reactivity. Unlike bulk commodity silanes, methylpropyldiethoxysilane plays a role in targeted surface treatments, additives in resins, water-repellent coatings, and specialty sealants. Its effectiveness comes from how easily it grafts to both organic and inorganic materials, thanks to its well-chosen side chains and alkoxy groups.
Methylpropyldiethoxysilane appears as a clear, colorless liquid at room temperature. Its molecular weight sits around 190 g/mol, and it boasts a fairly low boiling point in the 170-180°C range, kicking up a faint, sharp odor that signals both volatility and reactivity. The viscosity falls on the lower side, making it easy to handle in both batch and continuous processing. Although not defined by strong hydrogen-bonding, its alkoxysilane structure lends it partial solubility in organic solvents like alcohols, ethers, and to some extent, hydrocarbons. Water slowly hydrolyzes it, breaking ethoxy groups off and forming silanols, which quickly condense. The flash point hovers above many common plastics, so the safety margin for handling sits higher than some more flammable silanes. Storage in airtight, dry containers helps avoid unwanted hydrolysis and keeps the product fit for sensitive applications.
Typical technical documentation lists a purity over 98% for methylpropyldiethoxysilane. Manufacturers label the product with molecular formula C8H20O2Si, a CAS number for regulatory tracking, and safety data including recommended storage temperatures and moisture protection. Labels also require hazard pictograms under international law, flagging risks of skin and eye irritation. Many suppliers test for water content below 0.2% and set limits on key impurities such as chlorosilanes and unreacted starting materials. Properties such as refractive index and specific gravity make repeatable processing possible, and experienced operators lean on detailed spec sheets to tune process variables or flag inconsistent batches.
The standard way to make methylpropyldiethoxysilane involves a Grignard or alkylation route, starting from methylpropylchlorosilane. Chemists react this precursor with ethanol, using a catalyst such as acid or base, producing ethoxysilane and releasing HCl as a byproduct. In modern plants, process engineers focus on continuous purification to ensure the right ratio of ethoxy groups and to minimize unreacted chlorides or side products. These steps—hydrolysis control, careful distillation, and inert storage—make sure the product meets exacting industrial needs. From experience, quality control in silane manufacture never rests; even a small jump in residual moisture can spark downstream issues in coatings or resins, so production demands tight checks at every stage.
Chemically, this silane shows its usefulness through two faces: the moderately reactive ethoxy groups and the less-reactive methylpropyl side chain. On one hand, in wet conditions or in the presence of catalysts, the ethoxy groups hydrolyze, which allows the silane to cross-link with glass, metals, ceramics, or fillers. This forms durable, water-resistant bonds often needed in electronics encapsulants or adhesives. At the same time, the organic methylpropyl fragment gives extra flexibility in compatibility with non-polar substances. Chemists often tune the hydrolysis rate by using acid or base catalysts and can graft extra functionality onto the product for custom performance, such as UV stability or improved chemical resistance. The silicon atom stays at the heart of all these reactions, acting as a link between the inorganic and organic worlds.
Methylpropyldiethoxysilane appears under several chemical aliases, which helps buyers and scientists keep track as they cross language or brand boundaries. Alternative names like "diethoxy(methyl)propylsilane," "propyl(methyl)diethoxysilane," or their code numbers in global catalogs keep paperwork and inventory sorted. Each supplier usually stakes a preferred trade name or brand, but underneath the label, the chemical identity matches the standardized molecular structure.
Dealing with organosilanes means respecting a few clear safety rules. Methylpropyldiethoxysilane, like many of its cousins, calls for gloves, eyewear, and adequate ventilation to prevent irritation or accidental contact. In tight, hot spaces, workers need explosion-proof electrical equipment due to flammable vapor hazards. Leaks should be soaked up with inert absorbents; water makes the compound react, sometimes giving off heat and volatile byproducts. Standard firefighting gear covers most upset scenarios, but chemical foam is a better pick than water. Occupational hygiene centers on proper labeling, storage below 30°C, and routine drum inspection. Operators learn the lesson early—take the safety data sheets seriously, because even small spills or vapor leaks can drive costly downtime or require emergency cleanup.
Manufacturers turn to methylpropyldiethoxysilane when demands for adhesion, water-repelling performance, or surface modification get specific. In my experience, the coatings industry leans on it to improve how paints or protective layers cling to metal or glass. Plastics and rubber engineers use it as a crosslinking agent to get stronger, more flexible end products, especially for cables and seals exposed to extreme moisture or chemicals. Semiconductor plants rely on silanes in wafer passivation or for creating hydrophobic films on microchips. It even finds its way into modern optical fibers, acting as a coupling agent to keep layers united through years of use. Silane chemistry basically built the bridge between organic polymers and tough, long-lasting inorganic surfaces, and this compound keeps that bridge wide and stable.
Scientific curiosity about silanes only grew in recent decades. Researchers in academia and industry play with new modifications of the methylpropyldiethoxysilane backbone, searching for ways to fine-tune reactivity or introduce eco-friendly behavior. Green chemistry groups are trying to swap traditional hydrocarbon solvents for water or bio-based carriers during formulation, hoping to cut hazardous emissions during use or disposal. Materials scientists run experiments on silica nanoparticles or surface grafting, looking to expand the utility of these silanes in emerging fields like nano-coatings, flexible electronics, or self-cleaning surfaces. If a paper publishes on improved mechanical properties, manufacturers jump in to trial small-scale runs, often sharing results through technical conferences and patent filings. This constant churn of testing and feedback helps keep production standards sharp and inspires new product lines built on proven silane technology.
Toxicology profiles for methylpropyldiethoxysilane point to moderate acute toxicity, with main concerns about skin and eye irritation, as well as possible respiratory issues if vapors build up. Animal studies, while limited, pushed suppliers to tighten exposure limits and boost personal protective equipment on job sites. Regulatory bodies like the ECHA and OSHA set guidelines on exposure, with typical workplace limits based on both short-term peaks and long-term averages. The silane can break down in the environment or on contact with moisture, so risk assessments often focus on controlling emissions during production, use, and cleanup. Companies in the circle run routine training and exposure monitoring to catch hazards before they threaten worker health or trigger environmental liability.
Looking out to the next decade, methylpropyldiethoxysilane could find new homes in green construction, lightweight composites, or even biodegradable surface treatments—especially if the push for sustainable materials keeps gathering steam. Advanced manufacturing, especially around flexible displays, wearable tech, or EV batteries, calls for new silanes that punch above their weight in performance and safety. With regulators shining a light on environmental safety and worker exposure, R&D labs face real pressure to cut hazardous byproducts and expand life-cycle testing. From here, practical collaborations between suppliers, university labs, and end users look set to sharpen the way this silane gets made, handled, and applied. Whether it ends up in an experimental nanocoating or a standard high-voltage cable, its story keeps evolving as markets and scientists set fresh benchmarks for what a specialty chemical must deliver.
Methylpropyldiethoxysilane doesn’t show up in TV ads, but it plays a quiet role in making modern products tougher and more reliable. I’ve worked with chemists who swear by these compounds for sealing surfaces that don’t want to stick together. Most folks would never guess how often this kind of silane improves things in the background. Electronics, automotive coatings, construction adhesives—this compound lends a hand by prepping surfaces so coatings hang on better or making rubbers and plastics last longer under hard use.
Try sticking plastic to metal or rubber to glass without the right chemical help—it won’t last. Methylpropyldiethoxysilane acts like a matchmaker in composites, connecting materials that wouldn’t pair up on their own. I’ve seen how it adds a thin chemical layer that links two worlds, helping companies produce lighter, tougher products while keeping performance high. Without this, car dashboards might crack sooner, and electronic circuit boards could become less reliable. The bond it gives is more than just physical; it’s chemical, so things stay connected even under stress or in tough environments.
In construction, getting water out of the picture saves headaches down the line. Methylpropyldiethoxysilane finds its way into sealants and coatings that stop moisture from seeping into concrete or bricks. Friends working in building upkeep tell me that surfaces treated with silanes like this one need less repair, and the evidence is easy to see over time—walls stay cleaner, spalling drops off, and expensive overhauls become less frequent. The science? This molecule reacts with moisture on the surface, creating a water-repellent layer that helps bricks and stone fight off decay for years.
Tires, hoses, and window seals take a beating—sun, ozone, heat, wet roads. Additives based on methylpropyldiethoxysilane boost the bond between fillers like silica and the main rubber, adding life to everyday products. It’s a detail, but cars rolling down the highway and windows holding out rainstorms show how lasting connections lead to fewer breakdowns. Less waste, longer lifespans, and steadier performance are the real benefits. Companies test rubbers with and without these additives, and the difference in wear resistance and flexibility is obvious.
Every chemical has to face the pitfalls of environmental and health safety. Serious research follows the use of silanes across industries. Regulatory agencies keep a close watch—workers using the pure form wear advanced masks and gloves because breathing in vapor or skin contact could be risky. Waste treatment methods have come a long way, with industries investing in capturing unwanted byproducts before they hit the air or water.
There’s a push for safer substitutes and greener versions of specialty chemicals. Researchers develop new ways to use less of these substances or create blends with lower environmental impact. It helps when organizations share testing results and best practices, driving smarter, safer use across companies. My own work tracking technology updates has shown that transparency and investment in better chemistry keep safety and performance moving in the right direction for everyone involved.
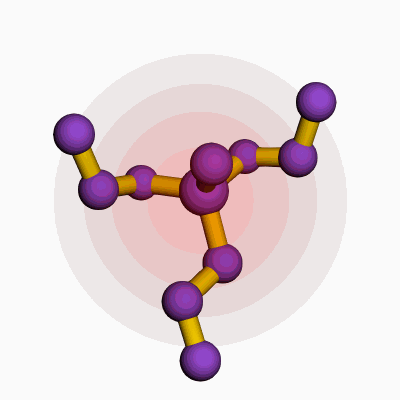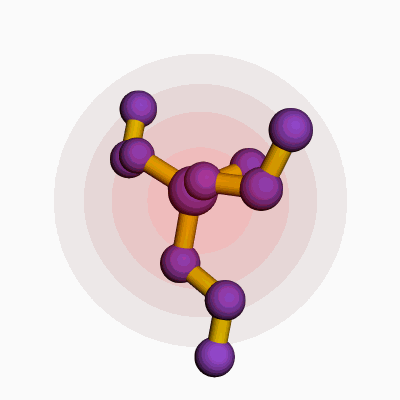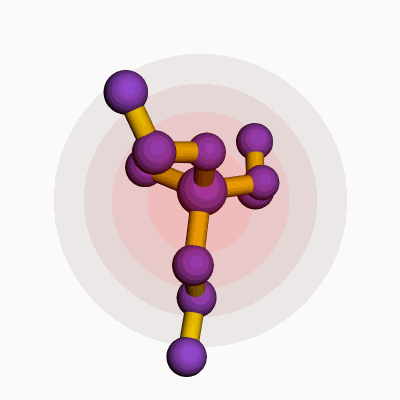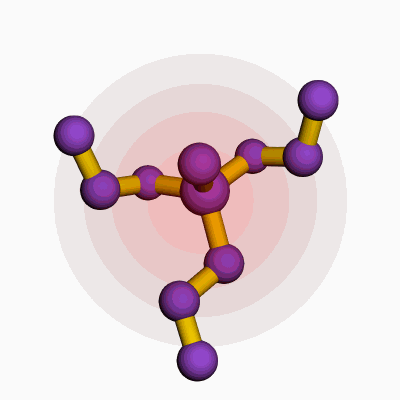
Methylpropyldiethoxysilane comes with a chemical formula of C8H20O2Si. This formula shows a combination of one methyl group, one propyl group, two ethoxy groups, and a silicon atom. Silicone chemists and manufacturers rely on the clarity of such structural makeup because even a single atom’s change can mean a wide gap in properties and applications.
The presence of two ethoxy groups in methylpropyldiethoxysilane grabs the attention of chemists working with surface treatments. These ethoxy groups mean the molecule can react with moisture in the air or on surfaces to form stable bonds. This is not just some theoretical property. In real-world applications like paints or coatings, the strength and durability that result from these bonds protect surfaces for years. Weak chemical structures in a silane can cause coatings or adhesives to fail, wasting time and resources for everyone from factory workers to homeowners applying sealants.
Because of the rise of health and environmental concerns, understanding what each part of a chemical like methylpropyldiethoxysilane does becomes more than just academic. Ethoxy groups can hydrolyze and release ethanol. Factories dealing with this chemical must ensure proper ventilation and handling, since ethanol vapor at high concentrations can affect both workers’ health and local air quality. Regulatory authorities such as the EPA pay attention to these compounds, so keeping tabs on formulas goes hand in hand with meeting safety standards. I recall a time working in a lab, where a forgotten, improperly sealed silane bottle started to seep vapors, which led the safety officer right to us. A lesson learned about chemical stewardship, which starts with understanding the formula.
Chemical names can get confusing, and for those in technical fields, using the wrong silane in a formulation can throw off an entire production run. Reliable chemical formulas, like C8H20O2Si for methylpropyldiethoxysilane, let professionals track ingredients from the lab straight to large-scale manufacturing. Mistakes with structure can lead to weak adhesion in electronics or improper curing in resins, triggering recalls and extra costs. As a chemist, I experienced project delays when a supplier sent a misnamed silane. Only close attention to the correct chemical formula let us spot the issue before things escalated.
Knowledge about the exact formula helps create safety protocols much easier. Workers can access the right protective gear and procedures when they know what compounds can be produced from methylpropyldiethoxysilane’s breakdown. Material safety data sheets rely on true formulas, not just trade names. Open communication across the supply chain, including accessible education and labeling, helps reduce accidents and costly mistakes. Chemical manufacturers, end-users, and regulators all benefit when formulas are correct and openly shared. This isn’t just about checking a box. It’s about keeping people safe, keeping equipment running, and building trust across the industry.
Methylpropyldiethoxysilane might not ring a bell for most folks, but it turns up in laboratories, materials science, and some specialized manufacturing. It’s one of those chemicals that lives on technical product labels and in the binders of safety sheets tucked away on factory shelves. With news about hazardous compounds and workplace accidents, it makes sense to wonder if this chemical poses much risk, either to health or the environment.
Chemicals like methylpropyldiethoxysilane don’t get much attention outside industry, but just because something isn’t a household name doesn’t mean it’s free of risk. The structure points to the family of organosilanes, which sees regular use as adhesives or additives. Many organosilanes, when they contact moisture, break down into smaller molecules. This property brings some risk. If spilled, as it meets water or even humid air, it may release ethanol. This gas can spread quickly and, without enough ventilation, catch fire. The National Fire Protection Association places some organosilanes in classes that need close attention due to their flammability.
Contact with skin or eyes can also bring trouble. Methylpropyldiethoxysilane can irritate, sometimes causing redness or burns. Industry data shows repeated or long-term exposure, even at low levels, might worsen symptoms. I think about entry-level factory jobs, where new workers might not recognize skin tingling as a sign of chemical exposure. Without gloves or goggles, small splashes add up fast. Not everyone stops to clean up, and that’s where injury starts. The strongest lesson comes from stories where basic safety steps got skipped, and a simple spill led to weeks of trouble.
Poking through academic studies and government safety sheets, real, immediate toxicity seems moderate. This chemical isn’t among the worst offenders, not as deadly as phosgene or cyanide. It doesn’t pile up in the body the way heavy metals do. Still, inhalation can inflame airways and trigger headaches or dizziness. Some organosilanes disrupt nervous system activity if exposure goes unchecked. Environmental groups log rare but real spills affecting aquatic life, as breakdown products drift through drains and outfalls. Most risk comes not from a rare splash, but from jobs where exposure happens again and again, shift after shift. It’s reasonable to think chronic low-dose exposure has yet to be fully mapped out.
Workplace safety officers focus on training and gear. Goggles, gloves, and strong ventilation systems beat paperwork. The U.S. Occupational Safety and Health Administration (OSHA) and Europe’s REACH program both set limits for workplace exposure and reporting. Factories need to keep chemicals well-labeled, clean up spills fast, and treat waste water so harmful substances don’t reach rivers. My own time running quality checks showed enough close calls with mislabeled containers to convince me robust storage and regular inspections are non-negotiable.
Even for small labs or custom manufacturing, sources like the Safety Data Sheet—easily found online—give straightforward do’s and don’ts for personal protection, storage temperature, and spill cleanup. Going beyond the letter of the law, site managers should run refresher training and double-check safety stock during inventory. Industry can learn from its own history of mistakes and near-misses. Regular reviews, hearing out junior staff who often spot hazards first, drives improvement quicker than memos ever do. Chemistry gives us powerful tools, but only if respect and caution lead the way.
Methylpropyldiethoxysilane isn’t a household name, but handling it safely can make all the difference in an industrial setting. Leaving it out on a shelf in the wrong conditions may seem harmless, but this stuff reacts fast with water and moisture in the air. Ignoring storage advice tends to lead to dangerous fumes or even a nasty spill, and nobody wants to clear up after a chemical chain reaction.
Heat is not a friend here. This chemical holds together just fine at lower temperatures, but exposure to heat speeds up unwanted reactions. Most storage rooms designed for volatile chemicals use climate control, and rightly so. I’ve walked into enough stuffy, overheated storage closets to know how quickly simple rules get ignored. Oddly enough, a forgotten thermostat setting often leads to the kind of phone call no safety officer wants.
Humidity deserves just as much respect. Moist air spells real trouble for alkoxysilanes like methylpropyldiethoxysilane. A dry, well-ventilated place keeps the worries at bay. We’ve all seen what happens to cans left near a leaking pipe—the label comes off, rust sets in, and liquid starts seeping. With this chemical, water means violent reactions and possibly hazardous byproducts. Dehumidifiers earn their place, especially during damp summer days.
Glass and high-grade stainless steel containers block out air and moisture well and don’t flinch at a little splash from a reactive substance. Polyethylene containers sometimes sneak into storage rooms since they look sturdy, but long-term use risks slow leaks or breakdown from chemical exposure. Sealing matters—screw caps beat snap-on lids every time.
Don’t forget the labels. Clear and tough, with every hazard symbol marked. I’ve seen too many people reach for an unlabeled bottle, thinking it’s just cleaning fluid. Labels serve as a quick reminder about what’s inside and how careful one ought to be. Every trainer’s lesson on chemical storage highlights this, and with good reason.
Chemical storage isn’t about fitting as much as possible onto one shelf. Any storage area should allow room between different chemicals. Methylpropyldiethoxysilane does not play nicely with strong acids, strong bases, and, most of all, water. I remember a story from a local plant where a cleaning crew set down a bottle too close to a leaky water jug—cost them a four-hour evacuation and a bill for hazardous waste cleanup.
Regulations set by OSHA and similar agencies offer practical guidelines. They don’t just fill binders with red tape—they back those rules with hard-earned lessons from past accidents. Employees get the right gloves, goggles, and lab coats for a reason. Spill kits nearby, regular safety drills, and day-to-day respect for the chemical’s quirks usually stop small mistakes from getting out of hand.
We live with stronger chemicals than ever, and a little attention to storage still makes the best safety tool. If everyone involved knows the rules and treats hazardous chemicals with healthy respect, people stay safe and the work continues.
Methylpropyldiethoxysilane, known in the industry for its use in specialty polymers, coatings, and silane coupling processes, isn't the type of chemical you see on a hardware store shelf. Safe handling comes first. I’ve been in plants where the air smells faintly sharp, and even simple tasks around these silanes show you how much a packaging decision matters.
Drums—usually made of steel or high-density polyethylene—show up on most loading docks, holding anywhere from 20 liters to 200 liters. These drums resist corrosion, seal tightly, and keep leaks away from warehouse staff. Folks unloading trucks can spot damage easily if a drum takes a knock during shipping. Some handlers want steel in case of accidental impacts, while PE works well in settings where weight adds up and moving a drum should not strain a back too fast.
Bulk customers step up to ISO tanks. I’ve seen these stainless steel monsters rolling on flatbed trucks and sitting under pipe racks. They carry thousands of liters, making them workhorses for large manufacturers blending batches round the clock. Seals, pressure relief valves, and careful washing help each tank stay safe and clean for the next load. Food and pharma operators worry about even tiny leftovers, so tank return and inspection routines keep everyone honest.
Fluorinated plastic containers fill a gap where you need resistance to moisture and don’t want your silane breaking down when it meets just a bit of water vapor. After all, silanes react with stray humidity. I’ve seen small shops opt for tight-head jerricans (5 to 25 liters) with tamper-proof closures, knowing that staff working outdoors or in a poorly ventilated shed need as much spill protection as the big guys.
Every packaging type comes with trade-offs. Price is never just for the container—you’re paying for safe floors, fire insurance bills, training, and lost product if a cap pops loose. Data from the European Chemicals Agency shows organosilanes can pose significant hazards if spilled, including flammable vapor clouds and nasty burns on skin. It pays to double-check that labels and seals hold up through rain, cold, and careless forklifts.
Customers with different needs push suppliers to get creative. A big paint company facing labor shortages might want 1,000-liter IBCs with tamper-evident seals, saving on drum handling and reducing downtime. An R&D lab ordering a few kilograms looks for UN-approved bottles that slide into chemical cabinets and survive shipping by courier.
Waste and environmental impact mean more now than even a decade ago. The best plants I’ve seen build closed-loop return programs, making sure drums and tanks come back, are checked for damage, cleaned as necessary, and sent out again. Multinational producers follow strict tracking: every empties gets logged and inspected before reuse, cutting both landfill waste and unauthorized disposal risks.
Smaller buyers often struggle. During conversations with logistics folks, the pain points are always storage space, disposal hassle, and minimum order size. Suppliers could help by offering flexible small-pack options and bundling in recycle or return services. It’s smart business, and it keeps both janitors and chemists happier. Regulatory pressures keep rising year by year, so early investment in better labeling, leak-detection closures, and safety guidance makes supply chains stronger.
In short, the real story sits with experience and adaptation. Packaging plays a bigger role than many realize—not just in product quality, but in keeping everyone safe and keeping a company’s reputation solid.
| Names | |
| Preferred IUPAC name | [methoxy(methylethoxy)oxy(propan-2-yl)silane] |
| Other names |
Diethoxy(methyl)propylsilane Methylpropylbis(ethoxy)silane Propylmethylbis(ethoxy)silane |
| Pronunciation | /ˌmɛθɪlˌproʊpiˌdaɪˌɛθɒksiˈsaɪleɪn/ |
| Identifiers | |
| CAS Number | 41230-42-4 |
| 3D model (JSmol) | `Methylpropyldiethoxysilane` JSmol 3D model string: ``` COCC[Si](C)(CC)OCC ``` |
| Beilstein Reference | 1741076 |
| ChEBI | CHEBI:88135 |
| ChEMBL | CHEMBL4296972 |
| ChemSpider | 20263406 |
| DrugBank | DB16689 |
| ECHA InfoCard | 0481b733-2f6c-41be-8774-ada8d94e3b8b |
| EC Number | 212-207-5 |
| Gmelin Reference | 86212 |
| KEGG | C18539 |
| MeSH | C07-H14-O2-Si |
| PubChem CID | 86772 |
| RTECS number | VV7310000 |
| UNII | 8Y71036G1C |
| UN number | UN2662 |
| CompTox Dashboard (EPA) | `DTXSID4060610` |
| Properties | |
| Chemical formula | C9H22O2Si |
| Molar mass | 220.38 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.856 g/cm3 |
| Solubility in water | Insoluble |
| log P | 1.98 |
| Vapor pressure | 0.6 hPa (20 °C) |
| Acidity (pKa) | 13.6 |
| Basicity (pKb) | 5.31 |
| Magnetic susceptibility (χ) | -73.0e-6 cm³/mol |
| Refractive index (nD) | 1.398 |
| Viscosity | 1 mPa.s |
| Dipole moment | 1.10 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 298.3 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | P261, P271, P280, P302+P352, P304+P340, P312, P305+P351+P338, P405, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 73 °C |
| Autoignition temperature | 270 °C |
| Explosive limits | Explosive limits: 1.1–9.7% |
| Lethal dose or concentration | LD50 (oral, rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, Rat: 8030 mg/kg |
| NIOSH | GVG768 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | No REL established |
| Related compounds | |
| Related compounds |
Trimethylethoxysilane Methyltriethoxysilane Propyltriethoxysilane Diethoxymethylsilane Diethoxypropylsilane |