
N-(2-Aminoethyl)-3-aminopropyltriethoxysilane (often called APTES or sometimes shortened to AEAPS) came about from a real need for better chemical adhesion and surface modification during the growth of organosilane technology in the 20th century. In the decades after World War II, chemists kept looking for ways to bridge the gap between inorganic world—think glass, metal, and stone—and the flexible realm of organic chemistry. Nobody likes when two things just won’t stick, and so came the push for organosilanes with multiple reactive ends. APTES came out of this push, with its dual amine groups and triethoxysilane end, and it solved a whole bunch of headaches for anyone trying to coat glass or stick things to metals robustly in industrial and research settings. Patents started cropping up in the late 1960s around its use in adhesion promoters and coupling agents, and from there, APTES found itself in paints, adhesives, sealants, and more.
APTES isn’t rare—this compound shows up in plenty of labs and factories. On the shelf, it mostly looks like a clear, pale-yellowish liquid. You’ll spot it in bottles labeled with names such as APTES, AEAPS, or the more mouthful “N-[3-(Triethoxysilyl)propyl]ethylenediamine.” Companies sell it under trade names like Silquest A-1120, Geniosil GF 94, and Dynasylan 1124. Popular sellers always stamp a purity (usually above 97%), as most applications won’t tolerate much extra water or impurities.
Pour some APTES out, and you’ll find a liquid with a faint but sharp smell. The boiling point lands around 260 degrees Celsius, and the density sits close to water—about 0.95 g/cm³. It mixes really well with most common organic solvents. You leave the bottle open, water in the air starts breaking down those ethoxy groups, so it gums up if exposed to moisture. The two amine groups bring in high reactivity; they can grab onto all sorts of other molecules, which really opens up the range for chemical tweaking. The silane end loves to react with surfaces rich in hydroxyl groups. Simple as it looks, it packs a punch at the molecular level.
Any drum or bottle of APTES carries clear hazard pictograms—there’s a corrosive symbol, and for good reason. The amine groups are tough on tissue, and the liquid gives off irritating fumes. Labels also stamp the CAS number 1760-24-3. Most technical data sheets note a refractive index around 1.428, and a flash point between 100°C and 110°C. Users crave up-to-date Safety Data Sheets (SDS), and you want to see info about shelf life, storage under inert gas, dryness, and sealed conditions to keep the stuff from wrecking itself before you open it.
Manufacturers put APTES together starting with γ-chloropropyltriethoxysilane through nucleophilic substitution reactions, swapping out that chlorine with the help of excess ethylenediamine. Sounds simple on paper but in practice it takes a knack for controlling moisture and byproducts. Too much water or an unclean reactor means you end up with sludge or even a polymeric mess that won’t do the job for surface treatment. The basic reaction isn’t complicated, but scaling it up for industrial work means investing in distillation and drying equipment just to keep APTES happy.
Chemists love APTES for its versatility. You can cure it onto glass, silica, alumina, or metal oxides. Once the ethoxy groups hydrolyze, the fresh silanols latch onto –OH groups on the surface, creating a covalent bond. That’s permanent. The two amine groups take part in further reactions, like linking with glutaraldehyde for biosensor creation or reacting with epoxy or anhydride functions in adhesives and coatings. I’ve watched it get tweaked by attaching dyes, drugs, or polymers to the amines, turning a basic silane into a specialized delivery vehicle or sensor anchor.
Open up a chemical catalog and you’ll find APTES listed under a stack of synonyms. Scientific names like N-(2-aminoethyl)-3-aminopropyltriethoxysilane, 3-(2-aminoethylamino)propyltriethoxysilane, and N-[3-(triethoxysilyl)propyl]ethylenediamine all mean the same stuff, though not every supplier uses the same label. Some simply call it aminoethylaminopropyltriethoxysilane, or just stick you with its abbreviation APTES. Brand names like Silquest A-1120 or Dynasylan 1124 occasionally pop up in industrial settings.
No one smart handles APTES barehanded. The liquid stings and burns skin, eyes, and lungs, so gloves, goggles, and lab coats stay non-negotiable. Fume hoods are needed, especially if you’re heating it or mixing it up with acids. Any spills mean quick action with plenty of water and a real focus on keeping it off skin. Storage means dry, cool areas, away from open flames and wet air. Industrial protocols demand eye-wash stations and strict occupational exposure limits, which regulators update on a regular basis. Emergency protocols aren’t filler; in my lab days, quick access to showers and good ventilation made more difference than any high-end instrument.
APTES shows up in places you might not expect. In biomedical research, it helps anchor biomolecules to glass slides for microscopy and sensor platforms for disease markers. Paints and coatings use it to bind colorfast dyes and pigments to mineral surfaces. Electronics makers value APTES for prepping silicon wafers and glass for microchips and display screens. Outside the clean lab, I’ve seen it used by restoration experts treating stone and concrete, boosting long-term water resistance and paint retention on sculptures and heritage buildings. The compound also acts as an adhesion promoter in fiber-reinforced composites and specialty adhesives, bridging the gap between synthetic polymers and inorganic fillers.
Researchers don’t let APTES collect dust. In my own experience, nanoparticle chemists treat silica dots or magnetic beads with a thin layer of APTES to make them “sticky” for further modification. It’s also been used as a key part of DNA microarrays, where stable immobilization makes or breaks a readout. Plenty of scientists have fiddled with APTES-derivative surfaces to control how cells interact with implants or sensors. Surface engineers keep hunting for tweaks that improve durability or target new groups, and the compound’s chemical flexibility keeps opening creative pathways. Publications keep pouring in—hundreds every year—spanning sectors from energy storage to tissue engineering, showing this molecule still sparks curiosity and invention.
Despite wide use, no one treats APTES as totally safe. Acute exposure causes severe skin and eye burns. Breathing in the vapor triggers strong respiratory irritation. Animal studies have shown moderate toxicity on ingestion, especially over repeated exposure. Chronic inhalation links to organ damage, especially the liver and kidneys. Regulatory agencies in the US and EU keep updating safety data, flagging special risk to workers without protective equipment. Waste disposal calls for tough controls—spill it down the drain and you risk pollution, especially in waterways where hydrolysis byproducts can disrupt aquatic life. I take it seriously whenever working with aminosilanes, as even a single exposure can ruin a day or worse.
People keep finding novel jobs for APTES, and I don’t see the trend fading. Advances in nanotechnology, biosensing, and polymer composites drive the need for ever-better surface chemistries, and this molecule’s two amine groups plus a silane tail leave it open to nearly endless chemical tweaks. More sustainable production methods remain a focus, with greener syntheses cutting down on solvent waste, and safer handling protocols continue to evolve. The medical world especially wants high-purity, ultra-low toxicity versions for implant coatings and drug delivery. As digital health and green tech keep growing, demand for smarter, more selective coupling agents will only increase. Scientists and engineers need thoughtful regulation and more public data on environmental impact, but the drive for stronger, more customizable interfaces between worlds—organic and inorganic—keeps APTES in the spotlight, both for its track record and what lies ahead.
Years ago, I worked in a lab that struggled to get glass, metal, and certain plastics to cooperate in coatings and adhesives. The biggest headache came from how easy it was for things to peel apart. Somewhere along the line, someone rolled out a small bottle labeled N-(2-Aminoethyl)-3-Aminopropyltriethoxysilane. Unwieldy name, right? Folks in the business often call it APTES, and suddenly, the team’s day took a different turn.
APTES acts like a matchmaker between vastly different materials. Imagine trying to get silicone and glass to behave as one in a high-performance sealant. APTES forms chemical bonds with both—etching itself into the surfaces, helping a coating stick for the long haul. In a lot of ways, it’s the molecular duct tape the modern coatings, adhesives, and composites world leans on. Without it, surfaces that get stressed—like parts in cars or aerospace—end up failing too soon.
In another job, I watched plastics processors pour bags of glass fibers and other fillers into polymers while trying to boost strength. Take polypropylene, common in car bumpers or appliance casings. It’s notoriously slick. Add glass fibers directly, and they sometimes don’t wanna stay put. Manufacturers sprinkle in APTES because it grabs onto the glass and the plastic at the same time. This approach does more than just keep the blend together. It helps car parts survive bumps, drops, and the wear-and-tear of daily use.
Researchers from Dow Chemical and Solvay documented improvements in mechanical performance and moisture resistance by using silane coupling agents like APTES in composites. That translates into lighter car parts that use less material, which consumers end up appreciating at the gas pump.
Anyone who’s opened a smartphone and seen all the tiny chips should realize glue alone doesn’t cut it anymore. Circuit boards marry metals and plastics, often needing a dependable interface. Chemists don’t toss APTES in by accident. It locks onto silicon chips and printed circuit boards, prepping those surfaces for coatings, or for a layer of insulation that just won’t budge. What this really means is devices can get dropped, jostled, or sit in humid environments, without connections failing.
European Union regulations push for more durable and recyclable electronics every year. Coupling agents help by letting parts hold longer and work in thinner, lighter packages.
Another corner of the world—biosciences—relies on APTES to prep glass slides or particles for experiments. Labs tweak surfaces with the amino groups in APTES to create spots that attract DNA or proteins, making it easier to run diagnostic tests. No magic here—just smart chemistry turning plain glass into a biological tool.
Materials like APTES help build safer cars, greener electronics, and better medical tests. They give industry a boost to innovate. I’ve always believed engineers, chemists, and factory teams ought to pay attention to safety, even with handy helpers like this. Silanes have real industrial benefits but can bring respiratory and skin risks if handled carelessly. Following safety data and protective practices, not cutting corners, lets innovation serve everyone better.
Growing up on a small farm, one thing always stuck with me — many products start to lose their punch once they get too hot or too cold. It’s true for everything from fresh milk to commercial chemicals. Most products hold up best between 15°C and 25°C. There’s a reason for this range. Heat can break down critical ingredients, making them less effective or causing them to clump up. Cold temperatures might create moisture inside containers, leading to possible spoilage or corrosion. If you want to avoid headaches, keep things in a temperature-controlled spot, away from windows and vents. I remember learning that a few degrees swing can make the difference between a job done right and a pile of waste.
When humidity creeps into your storage space, it brings challenges you can’t see right away. Some powders and granules absorb moisture, which can turn them lumpy and much harder to work with. Moisture also can trigger mold or speed up breakdown of sensitive ingredients, whether it’s a shelf-stable vitamin or a specialty fertilizer. Unless you have a room with solid humidity control, find a dry area and keep the containers tightly closed. I’ve seen people lose half a year’s inventory just because the seal on a bag failed after a big summer storm.
Many folks overlook the impact of light on product stability. Direct sun or even strong indoor lighting can fade colors, weaken vitamins, and change the chemistry of certain compounds. If you don’t already have storage cabinets or bins that block light, think about adding them. Using amber or opaque containers may seem simple, yet this step keeps fragile goods strong and reliable — something I learned after having to toss entire boxes of faded labels and sun-bleached chemicals.
Sturdy, airtight packaging makes daily life easier. Using a cracked lid just because it “mostly” fits can spell disaster over time. Product spills and cross-contamination happen more quickly than people realize. I’ve seen storage spaces that were neglected, with spilled powder building up near the baseboards. Not only does this waste resources, but it can attract pests or foul up other products nearby. Use containers built for the job, and label everything with purchase and expiry dates. Taking a few minutes to clean up messes each time you open storage keeps everything safer and longer-lasting.
Practical safety comes down to more than fire extinguishers on the wall. Store incompatible products apart — acids away from alkalis, for example — to avoid dangerous reactions. Chemicals or powders should never sit near food or break rooms. Training new team members to recognize what goes where helps a lot over the long haul. I wouldn’t want my own family to risk a simple mistake, so I make sure to double-check storage signs and keep the safety data sheets handy. Fast access in an emergency means you’re less likely to scramble in panic.
It’s easy to let older batches collect dust at the back of a storeroom. I’ve made it a habit to rotate stock regularly — oldest up front for quick use, new arrivals toward the back. Proper documentation lets anyone step in and find what they need fast, with no surprises about age or quality. Following these basics not only saves money, it keeps everyone safer on the job. The attention you give storage and handling today pays off in peace of mind down the road.
You can spot that long chemical name, N-(2-Aminoethyl)-3-Aminopropyltriethoxysilane, stuck on safety labels in labs and factories. People know it in the industry as APTES or silane coupling agent. I’ve spent my fair share of days in engineering labs, handling reagents like this, mixing and coating glassware for experiments or surface treatments. It’s easy to overlook these substances as just another bottle on a shelf, but it pays to read those safety data sheets and not get overconfident with “standard” chemicals.
APTES can irritate the eyes, skin, and lungs. Splashing a drop on bare skin leaves that weird tingling, sometimes a mild burn. A quick search on PubChem or GHS data sheets shows APTES doesn’t rank as a high-tier toxin, but it’s far from harmless. Inhaling its vapors, especially when heating or in poorly ventilated spaces, means coughing, throat pain, or headaches for sensitive folks. I remember one morning we forgot to switch on the extractor fans – the amine odor cut through the lab and left us all rubbing our noses. It sticks around, even after a good wipe-down.
Much of that comes down to the amine groups in its structure. Amines often irritate mucous membranes and skin. If you splash the stuff in your eyes, you risk lasting damage. Swallowing a bit by accident – not that anyone should be tasting chemicals – would likely lead to nausea or even toxicity. Prolonged skin contact can dry or burn, turning hands red and sore. Animal studies show only moderate acute toxicity, but long-term effects are less studied. Silane chemicals don’t build up in the body easily, though accidental chronic exposure isn’t something to ignore. No sense in rolling the dice with your health.
Walk through any production plant where glass fibers or electronics get treated and you’ll see barrels of APTES rolling around. Workers sometimes shrug off gloves or splash without thinking much of it. I’ve seen old-timers turn up their sleeves, grinning about the “light burn” after a spill. It isn’t smart. Repeated exposures stack up. Even short contact can cause dermatitis, and that’s a clue to take things seriously.
Proper ventilation counts. Never dismiss the value of simple measures: fume hoods, nitrile gloves, lab coats, and splash goggles. Good habits keep you safe—eye wash stations within reach, careful handling, sealed containers. Training matters. OSHA and the European Chemicals Agency both rank APTES as a substance that needs respect in industrial use, setting exposure limits and stressing PPE.
Beyond protocols, real safety comes from building a culture where people share near-misses and talk openly about risks. Technology can help. Automated dosing and mixing setups have cut down on splashes in recent years. Better labeling, automatic ventilation sensors, and routine safety drills also make a difference. I’ve found regular refresher sessions, not just onboarding, keep people sharp rather than complacent.
If you come across APTES on your workbench, treat it like you would any chemical with proven irritancy. Assume gloves are your best friend. Know your emergency steps and handle containers gently. Environmental disposal counts as well. Down the drain isn’t an option—catch spills, neutralize, and follow hazardous waste procedures.
Reliable, consistent safety practices outlast good luck every time. N-(2-Aminoethyl)-3-Aminopropyltriethoxysilane isn’t the most toxic thing in a lab, but it earns its warning symbols for good reason. With mindfulness, up-to-date training, and a bit of healthy respect, there’s little mystery left in the safe use of this compound—just day-to-day discipline and care.
Sodium chloride, known as table salt by most folks, goes beyond spicing up our dinners. This white, crystalline solid brings together sodium (Na) and chlorine (Cl), held tight by an ionic bond. One sodium atom gives up an electron to a chlorine atom, making both happy with their new charges. The resulting crystals show up as transparent cubes if you bother to grab a magnifying glass and look closely.
The compound shows a melting point of about 801°C—pretty high for something so familiar. Toss some salt on icy driveways, and you’ll see another notable feature: salt drops water’s freezing point. Roads stay safer in winter because of it. The compound dissolves in water with ease, splitting into sodium and chloride ions. This ability transforms salt into an electrolyte, powering up everything from kitchen science fair volcanoes to salty sweat that helps your body stay in balance on a hot day.
Salt’s taste isn’t the only reason humans chase after it. This colorless or white solid sticks to a cubic shape thanks to the way the sodium and chloride stick together in a crystal lattice. If you hold a salt crystal under a strong light, you might spot a slight shininess. Pound it with a hammer, and you’ll see it shatter neatly along the lines of its crystal structure.
Pour granulated salt onto a damp table and you’ll notice how easily it picks up water from the air. In humid places, clumped salt in the shaker reveals its hygroscopic nature. Table salt’s density clocks in at around 2.16 grams per cubic centimeter, and it’s odorless, which leaves no hint of its importance in your kitchen or in industrial plants around the world.
I’ve always noticed someone grabbing the salt at every meal, but salt’s reach goes deeper. It preserves food—pulling moisture out of meat to keep it from spoiling. In my family’s kitchen, nothing goes to waste as long as there’s enough salt around to rub into fish or pork.
In manufacturing, sodium chloride means business. It helps turn raw brine into chlorine gas and sodium hydroxide, both of which become the backbone of products like bleach and soaps. Large-scale ice removal in winter depends on this compound’s physical knack for lowering freezing points. That simple fact keeps roadways safer for drivers and helps keep businesses open when the weather turns nasty.
Health stands out as another arena where salt’s properties make a difference. Doctors track sodium levels to spot problems with blood pressure or dehydration. Too much, and heart health suffers; too little, and muscle cramps take over. The need for balance keeps researchers tuned in to sodium chloride’s effects.
Growing up near farmland, I watched plenty of salt scatter on icy driveways every winter. The runoff affects the soil, messing up local plant life. Problem-solving starts with smarter road salt application technology and alternative de-icers in sensitive areas—like calcium magnesium acetate, which is less harsh on the land.
In food preservation, lower-sodium substitutes using potassium chloride give families options that help reduce the risk of hypertension. On a larger scale, recycling waste salt from chemical plants finds new purpose in places like water softening. Tackling salt’s impact takes effort from individuals and industries alike, but the goal remains clear: keeping the benefits while reducing the harm to our surroundings and our health.
N-(2-Aminoethyl)-3-Aminopropyltriethoxysilane may sound like just another mouthful of a chemical name, but it plays a role in adhesives, coatings, and sealants thanks to its ability to act as a coupling agent. The trouble with any chemical in this category is that, if handled carelessly, it won’t just vanish down the drain—it can cause harm to humans and ecosystems along the way. Breathing its vapors or getting it on your skin could cause irritation. Pouring it away or tossing it with regular waste might end up polluting the water table or putting sanitation workers at risk.
Working in a research setting, I've found chemicals like this one call for respect, vigilance, and readiness. Even if a compound seems benign on paper or has no obvious smell, invisible harm can linger long after a spill or bad disposal decision. Colleagues sometimes take shortcuts—pouring leftovers into a sink or landfill-bound bin—thinking “just this once” won’t cause trouble. Years in laboratories have shown me firsthand that these mistakes don’t stay hidden for long. I’ve seen corrosion in pipes. I’ve seen regulators fine labs over improper disposal and require months of remediation.
Begin with the Material Safety Data Sheet (MSDS). This document gives crucial tips for the safe handling and disposal of most specialty chemicals. The sheet for N-(2-Aminoethyl)-3-Aminopropyltriethoxysilane gives a clear picture: don’t treat it like household trash. For small lab quantities, it’s best to seal the waste in a durable, clearly labeled container that resists leaks and corrosion.
Keeping incompatible chemicals apart is a must. I’ve seen people mix organosilane waste with acids or oxidizing agents, and the result ranges from fumes to dangerous pressure buildup. A solid habit is to collect this and similar wastes in separate containers and inform your local hazardous waste coordinator. Most universities and research centers have procedures for routine pickups.
Municipal waste services don’t have the capacity or equipment to filter out specialty chemicals in household trash or sewers. Licensed chemical waste companies align their procedures with strict regulations, following guidance like the Resource Conservation and Recovery Act (RCRA) in the United States. They have the right incinerators or neutralization capabilities, staffed by people who know the dangers.
At home, this means storing leftovers safely and reaching out to a local hazardous waste drop-off event or center. Staff will check the label and provide a solution that keeps the substance away from general landfills and waterways.
For most people, chemical handling seems like something for experts, but truth is, everyone can play a part. A careless moment with a bottle can turn into a toxic problem for a neighborhood or local wildlife. Prioritizing clear labeling, proper storage, and certified disposal means protecting health and the environment. Sharing knowledge through employee training or public workshops steers people away from the old “down the drain” habit.
When in doubt about a chemical, consult the manufacturer or university safety office. They often have partnerships with waste handlers and offer advice by phone or email. Staying informed and honest about what’s in that bottle makes a real difference—both for ourselves, and for everyone downstream.
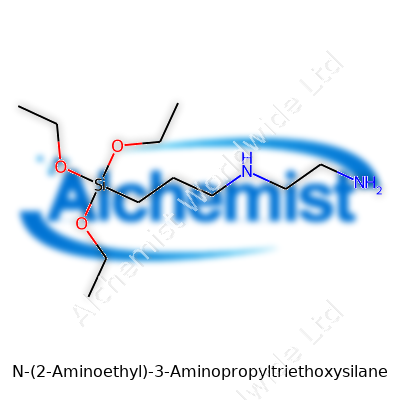

| Names | |
| Preferred IUPAC name | N-[3-(Triethoxysilyl)propyl]ethane-1,2-diamine |
| Other names |
N-(2-Aminoethyl)-3-aminopropyltriethoxysilane AEAPTES N-(2-Aminoethyl)-3-aminopropyltriethoxysilane N-[3-(Triethoxysilyl)propyl]ethylenediamine Aminopropyltriethoxysilane, N-(2-aminoethyl)- Triethoxy(3-(2-aminoethylamino)propyl)silane |
| Pronunciation | /ɛn tuː əˈmiːnoʊˌɛθəl θriː əˈmiːnoʊˌprəʊpɪl traɪˌiːθɒksiˌsaɪleɪn/ |
| Identifiers | |
| CAS Number | 1760-24-3 |
| 3D model (JSmol) | `3d_model.jmol.3D_daqwHonE1iwfQZKC35S8` |
| Beilstein Reference | 1424174 |
| ChEBI | CHEBI:85101 |
| ChEMBL | CHEMBL1376507 |
| ChemSpider | 21359548 |
| DrugBank | DB13790 |
| ECHA InfoCard | 03b920a9-8cfe-4f43-9797-41a0b21e9198 |
| EC Number | EC 217-164-6 |
| Gmelin Reference | 1570663 |
| KEGG | C18607 |
| MeSH | D017251 |
| PubChem CID | 2734163 |
| RTECS number | UF8790000 |
| UNII | A0N9UHO9E9 |
| UN number | UN3334 |
| CompTox Dashboard (EPA) | DTXSID6021397 |
| Properties | |
| Chemical formula | C11H28N2O3Si |
| Molar mass | 262.44 g/mol |
| Appearance | Colorless to pale yellow transparent liquid |
| Odor | Amine-like |
| Density | 0.973 g/mL at 25 °C (lit.) |
| Solubility in water | soluble |
| log P | -1.3 |
| Vapor pressure | < 0.1 hPa (20 °C) |
| Acidity (pKa) | 10.2 |
| Basicity (pKb) | 7.6 |
| Magnetic susceptibility (χ) | -7.3×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.437 |
| Viscosity | 10 mPa·s |
| Dipole moment | 6.3271 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 489.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -6237.6 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage. Causes serious eye damage. Harmful if swallowed. Harmful if inhaled. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07,GHS05 |
| Signal word | Danger |
| Hazard statements | H226, H302, H314, H317 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313, P301+P312, P330 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 113 °C |
| Autoignition temperature | 310°C (590°F) |
| Lethal dose or concentration | LD50 (oral, rat): 2995 mg/kg |
| LD50 (median dose) | 730 mg/kg (Rat, oral) |
| NIOSH | Not established |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for N-(2-Aminoethyl)-3-Aminopropyltriethoxysilane: "Not established |
| REL (Recommended) | 1 mg/m³ |
| Related compounds | |
| Related compounds |
N-(2-Aminoethyl)-3-aminopropyltrimethoxysilane 3-Aminopropyltriethoxysilane N-(2-Aminoethyl)-3-aminopropylmethyldimethoxysilane Bis[3-(triethoxysilyl)propyl]amine N-(2-Aminoethyl)-3-aminopropylmethyl dimethoxysilane |