
Chemists developed organosilanes to create bridges between inorganic materials and organic compounds. The interest in N,N-Diethylaminopropyltrimethoxysilane emerged during the push for improved adhesion between polymers and mineral surfaces in the late 20th century. As composite materials gained attention, research groups turned to compounds like this one to address weaknesses at boundary layers in glass-reinforced plastics and silicone rubber systems. In those years, researchers looked for silanes that did more than just stick stuff together; the hope was for better stability, easier processing, and higher reactivity toward functional groups. Lab notebooks of the era show tinkering with aminoalkyl groups, hoping the diethylamino unit would unlock better chemical coupling and broader application.
Chemists who handle N,N-Diethylaminopropyltrimethoxysilane see a clear, colorless to pale yellow liquid. It smells slightly amine-like, not overpowering, but definitely noticeable in a closed lab. Often housed in brown glass bottles or metal cans, this organosilane draws special attention for its dual roles—one end bonds to siliceous surfaces, the other sticks with organic systems. In industry, this material gets picked for its performance in improving surface properties, boosting adhesion, and introducing specific functionalities where standard silanes don’t measure up. The cost has come down from its early pricing, opening doors for producers in fields like sealant production, resin modification, and specialty coatings.
N,N-Diethylaminopropyltrimethoxysilane lands at a molecular weight around 235 grams per mole. Its density sits near 0.92 grams per cubic centimeter at room temperature. The boiling point sits above 220°C, so it handles moderate processing temperatures without rapid evaporation. The molecule features a trimethoxysilane moiety at one end and a diethylamino group at the other, spaced by a three-carbon propyl chain. It dissolves in most organic solvents but reacts vigorously with water, breaking down methoxy groups to release methanol and form silanols—a property that sometimes proves useful, sometimes bothersome. Hydroscopic tendencies mean bottles need to be tightened after every use.
Batches often carry a minimum purity of 97%, with color measured using the APHA scale—buyers expect values below 50 for most technical applications. Most manufacturers print CAS number 3069-29-2 on labels, with hazard pictograms flagging flammability and acute toxicity. Safety data sheets call out the risk of skin and eye irritation, with recommended PPE including goggles, nitrile gloves, and lab coats. Labels usually include recommended storage at 2-8°C, away from moisture sources and out of direct sunlight. Tracking lot numbers remains a must for quality management and regulatory checks.
The standard synthesis involves alkoxysilylation of N,N-diethylaminopropylamine with chlorotrimethoxysilane. This reaction runs under anhydrous conditions, with organic solvent media like toluene or dichloromethane. Chemists add chlorosilane slowly to the amine under stirring to control the vigorous reaction. Hydrogen chloride gas evolves, and neutralization steps follow to scavenge acid residues. After distillation and drying, the crude product is purified by fractionation. This whole process rewards tight control—water and air can ruin yields, so inert atmosphere setups and dry-glassware routines become habitual. Scale-up brings challenges with temperature spikes and exothermic behavior, which engineers address through reactor design and automated dosing.
Reactivity centers on the three methoxy groups attached to silicon. Water or alcohols easily cleave these to create silanol groups, which then bond covalently to metal oxides, glass, or ceramic surfaces. The diethylamino group provides nucleophilicity for further organic modification, sometimes reacting with activated halides or isocyanates to build up surface-bound structures. In coatings labs, N,N-Diethylaminopropyltrimethoxysilane modifies fillers so they stick better to polymer matrices, and in resin synthesis, it serves as a copolymerizable unit. Sometimes, chemists tailor-make hybrid silanes by swapping out amines or varying alkoxy groups to give unique reactivity profiles or thermal behaviors.
People in the business side of chemicals often roll out this compound under names like DEAPTS, Trimethoxy[3-(diethylamino)propyl]silane, or Silquest A-1120. Some companies lean on custom trademarks, especially for blends and catalysts. Global supply catalogs list these aliases, ensuring cross-reference by researchers who work across languages and markets.
Handling N,N-Diethylaminopropyltrimethoxysilane calls for the same respect as any reactive intermediate. The most immediate hazard comes from chemical burns and vapor exposure—skin and eye contact leave rapid, lasting impressions. Inhaled vapors tend to irritate lungs and mucous membranes, so fume hoods or local exhaust run constantly during transfers and mixing. Spilled material on the bench can etch plastic and damage coatings, and residue buildup can create slippery working surfaces. Workers must have spill kits within reach and receive annual training in containment and first-aid procedures. Local regulations often classify this product as a hazardous substance, triggering extra layers of documentation and waste handling requirements; these are important in industry, university, or small-scale shop.
Companies turn to N,N-Diethylaminopropyltrimethoxysilane where improved wetting, adhesion, or surface compatibility matters. In my early plastics jobs, we treated glass fibers with this silane to hold up better inside high-performance epoxy composites—finished boats, circuit boards, and aerospace panels all depend on that strong, chemical handshake at the interface. Some paints and sealants blend it in to boost weathering resistance or promote dispersion of fillers. Electronics manufacturers rely on it for making silanized wafers and insulating layers, finding consistent behavior at moderate thermal cycling. Sometimes, product developers tweak the organic end to fix stubborn compatibility issues with specialty rubbers or paints, turning a borderline adhesive into a high-value formulation component.
Since 2000, research into functional silanes like this one has grown steadily. Recent R&D centers on tweaking the amino group for new reactivity or adding polymerizable units for better integration in hybrid networks. Environmental scientists have studied ways to produce these compounds with greener solvents and safer byproduct handling. Patent activity shows interest from both academic and industrial groups—the former trying to push the boundaries with novel reactions, and the latter scaling up new variants for mass-market applications. Journal articles report on surface functionalization, anti-fouling coatings, and even biomedical uses, exploring every way this versatile molecule can bring added value.
Toxicologists have studied this class of organosilanes for local and systemic effects. Animal testing indicates moderate toxicity at high doses—the compound can trigger eye and respiratory tract irritation, along with reversible narcosis at major exposures. Long-term studies focus on the effects of inhalation and repeated dermal contact; results suggest risks remain manageable with engineering controls and personal protection. Chronic toxicity and cancer risk data continue to accrue, powered by modern in vitro models and cell-based assays. Environmental safety specialists watch for hydrolysis byproducts and methanol release during use and disposal, warning of aquatic toxicity in spill scenarios. Regulatory reviews continue to urge prudent handling and waste management, pending more complete ecotoxicity data.
Growth in demand for composite structures, advanced coatings, and functionalized particles suggests strong future prospects for N,N-Diethylaminopropyltrimethoxysilane. The shift toward sustainable manufacturing leads to ongoing work on bio-based feedstocks and lower-waste synthesis routes. Developers imagine new applications in adhesives, protective layers for electronics, and even emerging nanomaterials. Universities invest in partnerships with industry to tailor silane chemistry for flexible electronics and lightweight transportation platforms. Policy makers and regulators push for clearer labeling and harmonized safety standards, signaling a maturing supply chain and growing global footprint. Experience shows that informed research and careful stewardship help turn promising molecules into powerful tools across science and engineering.
If you spend time mixing things up in a lab or a factory, a name like N,N-Diethylaminopropyltrimethoxysilane has probably popped up. Scientists like to call it DEAPTMOS for a reason—who wants to write the whole name every time? It’s a specialty silane, not something you find under the kitchen sink. This compound carries a silicon atom at its core, three methoxy groups dangling from it, and a long tail that pokes out a nitrogen atom wrapped in diethyl groups. That chemistry lesson pays off for anyone who wants materials to stick together, repel water, or carry certain charges on their surfaces. I started running into DEAPTMOS after college, working on coatings that needed to stay clear, stick well, and not peel in a few months.
DEAPTMOS goes right into making glass, ceramics, and metals talk to plastics. Think of it as a chemical bridge. One end of the molecule grabs onto a surface—be it glass or metal. The other end sticks out like a little flag, changing how that surface acts. Want a pane of glass that resists fogging? DEAPTMOS helps films stick tight. Need to make an epoxy bond better to a metal part? This silane makes the join less likely to fail. There was a big push a few years ago to design electronics with lighter, more flexible parts. Without the right silane, those thin layers just peeled apart at the worst time. I learned to respect just how critical these “boring” chemicals are—the difference between a device that works and one that fizzles out halfway through its warranty.
The methoxy groups on this molecule jump into action with moisture around, swapping out for bonds directly to the surface—people call this “coupling.” Once it takes hold, the diethylamino group at the end plays a few tricks. On plastics, it can attract dyes, adjust electrical charge, or bump up resistance to grime. In composite materials—like fancy car panels or high-end sporting gear—the silane stops water from sneaking in between layers. Any failure here leads to delamination, where layers start to peel like old wallpaper. I remember a project testing different silanes: The samples treated with DEAPTMOS handled tough freeze and thaw cycles without falling apart, thanks to the tight chemical handshake it gave between glass fibers and resin.
Like many strong chemicals, DEAPTMOS deserves respect. Getting skin or eyes near the liquid means trouble—burns, irritation, worse. Proper gloves and a face shield become non-negotiable. Fumes can irritate lungs or trigger asthma. Disposal brings its own headaches—pouring it down the drain puts groundwater at risk. We kept our workspaces loaded with spill kits and spent plenty of time in safety training. One big part of staying safe: storing it right. A tightly sealed container, kept cool, keeps air and moisture away, cutting down on unwanted reactions. Regulators in the U.S. and Europe keep an eye on import, sale, and disposal for exactly these reasons.
DEAPTMOS opens doors to advanced coatings, durable adhesives, and cleaner interfaces in electronics. Nothing glitzy, but these solutions help make solar panels last longer and cars run better. If more companies took training on handling potent silanes seriously—and worked with smart waste management—the risks shrink. Investing in research for less hazardous coupling agents could also pay off in the long run. For now, though, careful, informed use turns DEAPTMOS from a risk into a reliable workhorse for building stronger, smarter materials.
N,N-Diethylaminopropyltrimethoxysilane rolls off the tongue about as easily as it splashes out of its canister—not at all. This stuff comes with a sharp, amine-like odor and tends to react with moisture in the air, releasing methanol. Right away, a few hazards pop up. Leaving it open or using containers that aren’t sealed tightly invites in water vapor and then you get a chemical cocktail that no one asked for. So, I always double-check that the lid’s on and the drum seal looks tight before closing up shop.
You keep this chemical far away from water sources because it actually breaks down when it meets H2O. The breakdown tosses out methanol—a flammable and toxic compound by itself. If your storeroom gets humid or if someone mops the floor and sloshes water too close to the drums, things can get ugly. Keeping the storage spot dry is not just some best practice, it’s about preventing leaks, chemical burns, and a whole lot of paperwork. It helps to use a dehumidifier in the storage area and to check regularly for condensation on walls and drums, especially if it’s a facility near the coast.
Anyone who’s dealt with silanes knows they don’t like heat. Temperatures above 30°C speed up hydrolysis, which is just a fancy word for the chemical tearing itself apart, again creating methanol and, depending on the container, overpressurizing the drum. Warehouses without air conditioning in the summer will see these chemicals go bad fast. If you’ve ever had to explain to a manager why an entire order got wasted because the storage shed hit 35°C for three days straight, you’ll understand why you want to keep this chemical in a cool, shaded place. If the drums get warm to the touch, it’s time to move them.
I’ve seen some folks try storing silanes in containers that react with the chemical. Stainless steel and glass usually hold up well, while ordinary steel starts breaking down sooner or later. Polyethylene and polypropylene containers do the job, but the right liner can add years to your storage. It’s not just about the bin, though—the shelving, the labeling, even the pallets must hold up to long-term exposure. A rusty corner or oily residue can start a chain reaction.
I once made the mistake of skipping goggles and gloves for a quick transfer. The fumes alone gave me a wicked headache, and the splash on my skin took real effort to wash off. Respirators, gloves, and goggles feel like a hassle but protect against those very real hazards—methanol vapors, skin burns, and eye injuries. Make it a rule: don’t shortcut PPE with this stuff.
A lot of facilities train new hires on basic chemical handling and stop there. This chemical demands more—real training that shows how to spot leaks, seal containers correctly, respond to accidental releases, and check the expiration date every time you pull a bottle off the shelf. Regular drills, visible instructions, and a culture that supports speaking up about safety issues make accidents far less common.
Safe storage and handling of N,N-Diethylaminopropyltrimethoxysilane come down to respect: respect for the dangers, and respect for your crew’s well-being. Dry air, low temperatures, good containers, and a little vigilance go a long way—so no shortcuts. Methanol isn’t just a byproduct, it’s a risk that lingers in the air and seeps through the cracks if you don’t prioritize safety every step of the way.
The name N,N-Diethylaminopropyltrimethoxysilane doesn’t exactly roll off the tongue. It’s a chemical often used in industries that deal with coatings, adhesives, and plastics. Some folks have probably never been near the stuff. For people who work in labs or around manufacturing lines, this substance comes up more than most would like.
This chemical acts as a coupling agent. In plain terms, manufacturers use it to connect molecules that don’t want to stick together, letting plastics and paints do their job. That seems pretty routine. But whenever you see a compound with three methoxy groups and a pretty complex structure, alarm bells should start ringing about safety and toxicity. Not all chemicals in industry have proven track records for safety.
Over the years, chemists keep learning more about how workplace chemicals affect human health. N,N-Diethylaminopropyltrimethoxysilane, like many silanes, can irritate skin, eyes, and airways. Just breathing in the fumes can sting. Liquid contact can leave burns if the skin isn’t protected. I’ve seen colleagues rash up just from a small splash before they had a chance to wash it off. Some report asthma-like symptoms after repeat exposure, and science is still catching up.
Some research says breakdown products of alkoxysilanes, like methanol, can enter the body during use. Methanol is notoriously toxic and damaging to nerves and vision, even at relatively low doses. So even if this silane formula isn’t as dangerous as known carcinogens like benzene, rules around its use make sense.
Factories aren’t playgrounds. Nobody wants upset workers or full-blown accidents. N,N-Diethylaminopropyltrimethoxysilane seems harmless on paper—after all, most people in the industry have worked around it without serious issues if they respected safety protocols. Take short cuts on gloves, goggles, or good ventilation, and trouble arrives fast.
Manufacturers and labs follow strict handling guidelines for these chemicals. The “Safety Data Sheet” (SDS) details hazards, ranging from skin corrosion to respiratory harm. Eye protection isn’t extra credit—it’s vital. Handlers need chemical-resistant gloves, proper storage, and ventilation. This isn’t overkill or unnecessary red tape; people have landed in clinics after careless exposure to chemicals much milder than this.
After seeing some close calls in my lab days, I grew cautious. Smart companies do routine air checks where chemicals like this are used. They provide staff with real training—more than a single poster on the wall. Making sure that emergency showers work and that someone knows what to do if a spill happens matters more than most realize.Doctors who understand occupational exposure can catch early symptoms of chemical-related illness. Occupational health clinics ask pointed questions about what people actually use, not just what’s on the company’s safety report.
It’s not about scaring people away from useful molecules. Chemicals like N,N-Diethylaminopropyltrimethoxysilane help make stronger products that we all use daily. But no desk job or process line is worth risking permanent health problems. Reading up on the latest findings, updating safety measures, and catching health changes before they become serious—this is what responsibility looks like in modern industry. Never trust a substance just because it’s common.
I’ve seen a lot of specialty chemicals come and go, but N,N-Diethylaminopropyltrimethoxysilane (often shortened to DEAPS or something similar in a chemist’s lab notebook) actually brings something useful to coating formulations. This isn’t your average ingredient; it acts as a sort of go-between for organic and inorganic materials. That matters because paints and protective coatings rely on solid interactions with the surface they cover—otherwise, it all flakes off once weather or time does its work.
No one paints a wall covered in dust. In much the same way, industrial coatings need a “clean handshake” between the surface (say, glass, metal, or ceramic) and the coating itself. DEAPS contains both an amine group and reactive silane part. Once you add it to a coating formulation, silane groups react with surfaces— often after a touch of humidity or a controlled curing step—forming a strong chemical bond. That means the paint doesn't just stick on top, it really grabs hold.
This bonding step isn’t just chemistry trivia—companies rely on it to hit specific performance targets. A coating enhanced with DEAPS can stand up to harsh industrial cleaners, humidity, and temperature changes. Once, I watched a batch of treated panels run through a salt spray test in a paint lab. The panels with the silane modifier looked new after a week; the untreated ones, not so much.
Coating formulators rarely work with pure DEAPS out of the bottle; usually, it’s blended into resins or water-based solutions. Adding it in the right sequence matters. If you toss it straight into water, you might see clumping before it gets to react with the surface. Stirring it into a resin or solvent ensures it goes where it’s needed. Many shops have staff who’ve learned—sometimes the hard way—that the wrong mix order brings headaches later.
DEAPS doesn’t just boost adhesion. The amine part can help coatings resist corrosion and make surfaces more compatible with adhesives and sealants. In electronics, for example, it helps protective coatings cling to circuit boards, extending the life of expensive equipment. The same principles apply in automotive manufacturing and construction materials, where weather and chemicals test even the toughest coatings.
Silanes deserve respect in the workshop. Like many organosilicon compounds, they can release methanol when reacting with water. Ventilation and basic protective equipment are a must. I’ve seen some manufacturers invest in closed systems and better training, keeping risks low for workers and the wider community.
There’s industry pressure for coatings that last longer and impact the environment less. DEAPS gives chemists a tool for that job, helping paints grip surfaces without always reaching for heavy-metal additives. Regulatory agencies pay close attention, so responsible stewardship matters. That means using just enough of these silicon-based helpers to get the job done, exploring better waste handling, and always keeping an eye on safer alternatives.
Anyone who has juggled specialty chemicals knows that shelf life creates real challenges. N,N-Diethylaminopropyltrimethoxysilane, with its mouthful of a name, brings a set of quirks familiar to those dealing with organosilanes. Most bottles come stamped with an 18- to 24-month expiration, provided the cap stays tightly sealed, light is low, and storage hovers below 25°C. Pushing past two years simply risks wasting money or time. The chemical’s fate turns faster in humid or warm storage rooms—the trimethoxy groups start to react with water vapor, and users end up with unwanted silanols or even gunky polymers.
Reliable outcomes depend on reagent quality. Fresh N,N-Diethylaminopropyltrimethoxysilane does the job: it reacts cleanly, forms strong bonds, keeps coatings smooth, and lets adhesives stick the way they should. Old or hydrolyzed batches introduce all sorts of headaches. Surfaces fail to bond, textures change, and the whole process gets unpredictable. I’ve seen a lab lose a week’s work because someone grabbed an old bottle nobody checked—it had yellowed, thickened, and barely reacted. Chemists had to go back, clean up, and redo everything with a new bottle. Keeping track of not just the date on the label but any odd smells or colors gives a better picture than paperwork alone ever will.
The trimethoxysilane part hits the wall fastest. Exposure to moisture cracks those methoxy groups open, forming methanol and silanol byproducts. No amount of wishful thinking reverses this. Warm air, open bottles, and old packaging only speed up the decline. In practice, the bottle turns cloudy, lumps form, and a faint odor creeps in. We don’t just face a drop in purity—the whole reactivity profile shifts, undercutting work in coatings, adhesives, and surface modification.
Anyone who has spent time in a busy lab learns to fear the “unknown bottle on the back shelf.” Keep the containers closed tight. Avoid opening bottles in humid rooms. Decant what’s needed, then get that cap back on right away. Store in a cool, dry spot, far from sunlight—forget the cluttered chemical cupboard near the sink or heat vent. Label everything with the opening date. For shops using large quantities, smaller aliquot bottles cut down on repeated exposure, and save on costs over time. Smart buying—never order way more than needed—avoids rushing to use up stock before it spoils. These habits spare you not only wasted money, but also the trouble of starting over.
The average user may not run full analytical purity checks, but visual and olfactory cues matter. Cloudiness, thickening, or weird smells deserve caution. Better to pitch a compromised batch than risk a failed run or contaminated product. When in doubt, call the supplier. Most trustworthy vendors detail shelf life and recommend storage practices right on the safety data sheet (SDS), not just to shift blame, but because the chemistry truly changes after months at the wrong temperature or humidity. Reviewing real-world outcomes, as well as academic and industry sources, confirms what you see in the lab: N,N-Diethylaminopropyltrimethoxysilane works best when fresh and dry, and bad storage throws good money after bad.
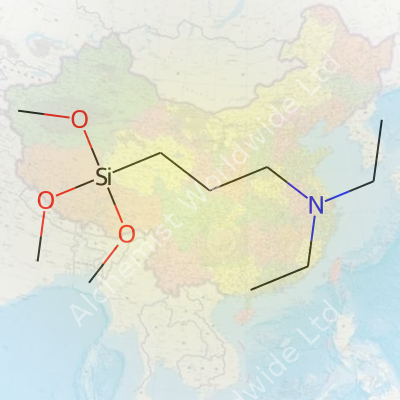
| Names | |
| Preferred IUPAC name | 3-[Diethyl(amino)propyl]trimethoxysilane |
| Other names |
N-[3-(Trimethoxysilyl)propyl]-N,N-diethylamine 3-(N,N-Diethylamino)propyltrimethoxysilane Diethylaminopropyltrimethoxysilane DEAPS 3-(Diethylamino)propyltrimethoxysilane |
| Pronunciation | /ˌɛnˌɛn daɪˌɛθɪlˌæmɪnoʊˌproʊpɪl traɪˌmɛθɒksi saɪˈleɪn/ |
| Identifiers | |
| CAS Number | 15180-47-9 |
| Beilstein Reference | 1461064 |
| ChEBI | CHEBI:60727 |
| ChEMBL | CHEMBL1544941 |
| ChemSpider | 63262 |
| DrugBank | DB13720 |
| ECHA InfoCard | 100.228.266 |
| EC Number | 203-934-1 |
| Gmelin Reference | 79142 |
| KEGG | C10629 |
| MeSH | D017225 |
| PubChem CID | 2734165 |
| RTECS number | YV9620000 |
| UNII | 9H7M0MM1TI |
| UN number | UN3334 |
| CompTox Dashboard (EPA) | DTXSID90944798 |
| Properties | |
| Chemical formula | C10H25NO3Si |
| Molar mass | 263.43 g/mol |
| Appearance | Colorless to yellowish transparent liquid |
| Odor | Amine-like |
| Density | 0.89 g/mL at 25 °C(lit.) |
| Solubility in water | Soluble |
| log P | 1.2 |
| Vapor pressure | 0.4 hPa (20 °C) |
| Acidity (pKa) | 10.2 |
| Basicity (pKb) | 4.8 |
| Magnetic susceptibility (χ) | -72.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.414 |
| Viscosity | 2.5 mPa·s (25 °C) |
| Dipole moment | 3.19 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 228.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02, GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H311, H314, H332 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313, P501 |
| Flash point | 74 °C |
| Autoignition temperature | 290 °C (554 °F; 563 K) |
| Lethal dose or concentration | LD50 (Oral, Rat): 1780 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2413 mg/kg |
| NIOSH | Not established |
| PEL (Permissible) | Not established |
| REL (Recommended) | Not Established |
| Related compounds | |
| Related compounds |
3-Aminopropyltrimethoxysilane N-Phenylaminopropyltrimethoxysilane N-Methylaminopropyltrimethoxysilane N,N-Dimethylaminopropyltrimethoxysilane N-Ethylaminopropyltrimethoxysilane |