
Octylmethyldimethoxysilane didn’t appear overnight. For decades, chemists poked and prodded organosilanes to figure out how silicon-based compounds could make surfaces water-resistant and more durable. Through the 1960s and 1970s, custom silanes showed up in research papers before anyone brought them into mainstream manufacturing. My first exposure came from a tech at an old adhesives lab, singing the praises of how silanes let construction materials laugh off water. In the 90s, builders wanted facades and tiles that shrugged off grime and freeze-thaw cycles, and here, Octylmethyldimethoxysilane started earning its keep. Industries recognized the value of tweaking simple building blocks to get the right balance between performance and cost. It’s been a quiet, iterative journey, but that’s pretty typical for chemical specialties that need to both solve a problem and fit into existing supply chains.
Octylmethyldimethoxysilane shows up as a clear, nearly colorless liquid with a faint, sharp odor. Companies delivering construction chemicals, waterproofing agents, and coatings prize it for its ability to bond decorative and structural surfaces to water-repellent layers that last. Automakers and electronics designers started using specialty silanes to add weatherproofing where rubber and plastics couldn’t compete. Compared to older silanes, this molecule’s longer octyl tail handles jobs where extra water or oil resistance matters. Instead of slapping on an extra coat of something, designers now grab this compound to build-in durability at the molecular level.
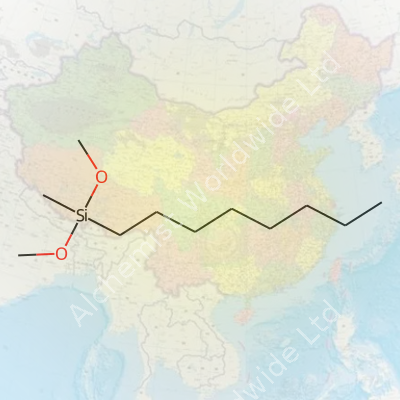
You can spot Octylmethyldimethoxysilane by its boiling point, hanging around 230°C under normal air, and a density just below that of water. Moisture in the air sends this silane into hydrolysis, so suppliers ship and store it under dry, sealed conditions. Its molecular formula, C12H28O2Si, balances a silicon core with two easy-to-cleave methoxy groups and a hydrophobic octyl chain, making it a smart choice for modifying both organic and mineral substrates. Under the right conditions, it leaves surfaces with a layer that water and oil both avoid. Chemists look for those telltale Si-O and Si-C bonds by IR and NMR to confirm they got the synthesis right.
In a warehouse or lab, you’ll find drums and jugs marked with CAS number 3069-40-7. Labels warn of the flammable and irritant nature. Purity runs 98% or higher for tech grades. Some companies add stabilizers to stop pre-mature hydrolysis. The packaging keeps out sunlight and locks out air, with documentation trailing every shipment from source to field jobsite. I saw once how a sloppy label can throw a repair crew off schedule, so robust tracking and safety sheets travel with every unit shipped.
Industrial-scale production tackles hydrosilylation. Chemists merge octylmethyldichlorosilane with methanol under controlled heat and pressure. They drive off the hydrochloric acid byproduct, dry the solution, and collect out the pure silane. Even a whiff of water knocks this process off track, so strict no-moisture rules sit at every stage. Small batch or lab makers start from scratch, controlling time, temp, and solvent quality, but factories follow a chain for efficient, reproducible output. This synthesis lets engineers dial scale up or down depending on real-world demand.
I’ve watched engineers tweak Octylmethyldimethoxysilane to fit all sorts of projects. When the methoxy groups break off in humid air or in a water bath, they let the silane form tough Si-O-Si bridges with glass, cement, or ceramic surfaces. Sometimes, R&D pros swap the octyl chain for other alkyls to suit different environments—like shorter chains when breathability matters, longer ones for oil-heavy settings. Adding special catalysts bumps up reaction rates. Some researchers are exploring dual-functional silanes that join the octyl group with a second reactive site to link up with new polymer chemistries. Adaptation keeps this compound relevant in a competitive market.
You’ll hear this stuff called Octylmethyldimethoxysilane, n-octylmethyldimethoxysilane, or by product names like Dynasylan® 1-Octyltrimethoxysilane (despite a slightly different structure), Silquest A-1230, or Momentive’s offerings. The synonyms often trip up first-timers, which leads to plenty of calls between R&D, purchasing, and site managers. I’ve watched vendors argue about which name matches the actual molecular structure. For any project, check the CAS number and read up to avoid mix-ups.
Teams mixing or applying Octylmethyldimethoxysilane need gloves, goggles, and a well-ventilated setup. The liquid can irritate skin, eyes, and airways, and it doesn’t play nice with open flames. Fire codes in most regions treat it as flammable. I once sat in a site safety briefing where one mistake—leaked vapor mixed with a spark—shut down a day’s work. Modern storage means double-sealed containers, spill kits nearby, and proper disposal according to government waste rules. Workers follow chemical hygiene plans and keep detailed logs for any accident. A training gap can make the difference between safe prep and a messy disaster, so ongoing education remains key.
Octylmethyldimethoxysilane covers a wide territory. In construction, it puts a virtual raincoat on concrete, bricks, and stones. Road crews treat highways to prevent freeze-thaw breakdown. Electronics companies use it as a primer to keep moisture away from circuit boards. Coatings producers look for ways to add anti-graffiti barriers or smudge-resistance to glass and screens. Textile makers chase new waterproofing effects that last through wear and washing. Sometimes you’ll spot it in marine and automotive paints for extra corrosion resistance. Hands-on technicians like it for its performance, chemists like its versatility, but end-users notice how surfaces stay cleaner and tougher in bad weather or heavy use.
Research rarely pauses. Current efforts focus on lowering the environmental impact—reducing volatile organic content, finding greener synthesis routes, and making sure the finished products break down safely at end-of-life. Some scientists want faster-curing or lower-temp versions for sensitive process needs, like electronics packaging. Others look for blends with nanoparticles, making superhydrophobic surfaces without sacrificing transparency or breathability. Labs collaborate with field crews to see how treatments hold up under real-world sun, salt, and abrasion. From start-ups to global conglomerates, investment in improving silanes keeps growing, especially as climate change demands longer-lasting materials with less waste.
There’s no shortcut to safety. Toxicity studies so far show Octylmethyldimethoxysilane as a low-hazard compound if handled right. It can irritate eyes and skin, and inhaling much vapor leaves you coughing—so safety teams stress protection and quick cleanup. Environmental monitoring looks at breakdown into harmless silanols and alcohols under sunlight and soil microbes. Some concern exists around manufacturing byproducts, but overseers track discharge at every step to keep water tables and air clean. Regulators eye new data all the time, updating permissible exposure limits and environmental release standards as new research rolls in.
Octylmethyldimethoxysilane isn’t fading out soon. Market demand for longer-lasting, lower-maintenance buildings, vehicles, and consumer goods gives this silane a stable home. Scientists keep searching for better performance with a smaller carbon footprint, and building codes now push for higher durability with less reapplication. There’s a rising call for biobased silanes, or versions that perform well under new recycled-material regulations. Businesses chasing green certifications and lifetime cost savings look to silanes like this one as a silent hero that stretches product lifespan without big investments in new infrastructure or labor. Ongoing education, lab investigation, and clear communication will push this compound and its relatives into more corners of industry and daily life.
Octylmethyldimethoxysilane pops up in places most people never think about, yet it plays a real role in shaping how products perform in daily routines. It belongs to a group called silanes, which help link things together at the molecular level. In plain language, it acts like a matchmaker between materials that do not naturally get along—like oil and water. Out in the world, companies use it to coat glass, concrete, textiles, and electronics, boosting how these products hold up against rain, sweat, and stains.
Picture an old stone facade darkened by years of street grime or a glass shower wall covered with water spots. Octylmethyldimethoxysilane changes what happens on those surfaces. It connects with minerals in stone or glass and forms a layer that keeps out water. This effect extends far beyond how things look. Buildings stay standing longer, require less upkeep, and resist damage from freeze–thaw cycles. Those concrete roads and bridges covered in graffiti or salt stains? Many of them have already soaked up silane products for defense.
Busy families rely on phones that bounce around in bags, headphones dropped in puddles, or smartwatches braving sweaty hikes. Silanes play a backstage role in making these gadgets last. Manufacturers use octylmethyldimethoxysilane to coat electronics, helping them repel moisture. That line of defense matters. According to the Consumer Technology Association, water damage sits near the top of reasons people replace phones. By sealing components, companies stretch the life of devices and cut down on urgent repairs and electronic waste.
A fresh shirt that shrugs off a rainstorm or a carpet that won’t hold onto juice spills owes a lot to water-repellent treatments. Octylmethyldimethoxysilane forms part of the recipe for these coatings. In my own experience, hiking through the woods while rain pours down feels less like a gamble when wearing a treated jacket. The water beads up and rolls away. Textiles keep their color and texture longer. Households with kids or pets see fewer stains, and cleaning tasks shrink to a minimum.
Talking about chemicals raises fair questions about safety. Octylmethyldimethoxysilane, like many modern additives, faces careful review in Europe, the US, and Asia. Manufacturers submit toxicology data, and agencies lay down rules for use. According to regulatory filings, no major red flags have turned up for this molecule when used as directed. That said, responsible handling in factories and attention to disposal matter for workers and communities. People expect companies to share clear information about what goes into consumer goods. Trust depends on clear labeling and good faith from both sides.
Today, sustainable choices sit high on the agenda. Scientists hunt for molecules that get the job done with less risk to people and ecosystems. Many firms look at renewable sources, biodegradable options, or lower-toxicity blends. Some limits appear—bio-based coatings don’t always match performance in extreme conditions. Still, the momentum for greener chemistry keeps growing. Companies willing to invest in research and honest communication will shape what protection means for the next generation of surfaces—both inside the house and out along the street.
Octylmethyldimethoxysilane pops up in plenty of manufacturing spaces, especially where water repellency and surface modification matter. This silane compound helps coat glass, ceramics, and stone. Its job is to protect, make surfaces resist moisture, and preserve materials that deal with tough environments. Most folks never hear about it, but for workers in factories or labs, Octylmethyldimethoxysilane is more common than coffee breaks.
My own background involves years in a small industrial lab, where chemical safety decisions shape how comfortable people feel stepping into work each day. Octylmethyldimethoxysilane carries some hazards worth respecting, and ignoring a material’s safety profile only takes one bad reaction to regret.
According to data sheets from trustworthy suppliers and chemical safety authorities, this compound can irritate skin and eyes. One time, our tech spilled a small splash on his wrist. It didn’t seem too harsh at first, but redness followed soon after, with an uncomfortable, itchy feeling. Quick washing with soap and water made things better, but no one in the shop ignored glove rules again. Vapors from silanes don’t mess around either. Inhaling fumes, especially if heating or mixing the liquid, leads to coughs and chest tightness for some people.
The GHS (Globally Harmonized System) classifies Octylmethyldimethoxysilane as an irritant. OSHA highlights the need for eyewash stations and strong ventilation. The European Chemicals Agency stresses that exposure limits exist for a reason. Even though the chemical isn’t considered acutely toxic under regular conditions, it does have the potential to damage tissue if misused or handled carelessly. Most responsible workplaces enforce protective equipment: gloves, goggles, face shields if splashing might happen.
Staying safe around Octylmethyldimethoxysilane comes down to building good habits. The rules sound simple—use nitrile gloves, keep goggles snug, run a fume hood when pouring or mixing—but skipping any step adds real risk. I’ve seen veteran workers forget, focusing on speed, only to end up with headaches and irritation for the rest of their shift.
Training helps, for sure. But what really changed the game in our shop was a visible culture: quick safety meetings, supervisors who double-checked ventilation, and a team that looked out for each other before firing up new batches. Skin checks happen before lunch, not just before clocking out. Containers stay labeled, and anyone can call for a pause if something smells odd. We also kept the material safety data sheet near the bench, not buried in a desk.
Chemicals like Octylmethyldimethoxysilane carry benefit and risk side by side. They make products last, strengthen surfaces, protect against weather and grime, yet carelessness hurts fast. Taking time for real safety habits, sharing lessons learned, and reading new reports or safety research keeps people better protected. Listening to stories helps too: most changes in our approach grew out of near-misses or seeing a coworker patching up a burn. No miracle cure exists, but a steady routine, strong information, and looking out for each other makes all the difference.
Octylmethyldimethoxysilane doesn’t get the press that some chemicals do, but it deserves a close look—mainly because it’s not as forgiving as folks would wish. Being a silane-based compound, it’s volatile and flammable. I learned pretty quickly in lab life that this isn't something you want laying around by a heat source or near open flames. A swing in temperature sometimes leads to pressure buildup in sealed drums. That’s not an accident anyone wants under their watch. Keeping it in a tightly closed, clearly labeled container, away from sparks, flames, and direct sunlight should be standard. Most places use temperature-controlled storage, sticking to a cool, well-ventilated space to reduce air humidity, since moisture will mess with this chemical fast.
Anyone dealing with Octylmethyldimethoxysilane quickly learns that water ruins the party. The reason relates to its chemical structure; any contact with water triggers hydrolysis, releasing methanol and leaving behind a sticky mess. That can cause moisture damage to packaging, hazards in the workplace, and ultimately wasted product. From personal experience, keeping desiccators or silica gel packs close by helps reduce moisture risk. A dry atmosphere inside the storage room pays off in the long run, preventing surprise reactions and broken seals.
Health and safety haven’t always gotten enough attention in chemical storage. I’ve seen what short cuts bring—eye and skin irritation, or worse. Volatile organic compounds like Octylmethyldimethoxysilane can vaporize at room temperature and cause headaches and respiratory problems if they hang in the air. Ventilated storage and handling spaces make a difference. Respirators, gloves, and safety glasses should be more than suggestions. They keep the hazards out of your body, so you can go home healthy.
No one gets excited about spill kits, but if you store Octylmethyldimethoxysilane, you appreciate their value once you’ve put one to use. A little carelessness can lead to slippery floors, and because this stuff can evaporate fast, contaminated air follows. Quick absorption with sand, earth, or commercial absorbents works, but don’t ever wash it down the drain. Training employees on proper clean-up, along with having the right fire extinguishers on hand, fits into any responsible storage plan. Local regulations call for reporting larger spills, and the faster you move, the less the impact on people and the environment.
Anyone who’s opened an unlabeled drum knows the risks. Regulatory bodies expect clear hazard communication, and anyone who values their team’s safety follows through with proper pictograms, date codes, and hazard phrases. Europe’s REACH and the US OSHA offer clear rules, and they protect more than the bottom line. Documented tracking, first-in-first-out rotation, and routine inspection all limit surprises and prevent expired or degraded material from slipping into production.
Like a lot of hazardous chemicals, Octylmethyldimethoxysilane doesn’t forgive mistakes. Automation and closed transfer systems have started to make a dent in workplace exposures. I’ve noticed larger operators using smart inventory systems to monitor not only stock levels but tampering, temperature, and humidity—adding peace of mind. Anyone managing this chemical owes it to their crew to review safety protocols regularly, include practical training, and invest in reliable storage gear. That’s what builds trust, keeps people safe, and reduces downtime from accidents or poorly handled stock.
Every time I’ve looked for a hydrophobic solution in construction or paint, Octylmethyldimethoxysilane comes up. Many concrete coatings count on this compound for its water-repelling magic. Spraying it onto walls or sidewalks, the surface picks up a resistance to moisture that slows down water damage and fend off stubborn stains. Contractors love this because it cuts down on long-term maintenance.
In paints, chemists add it before the last blend step. Careful mixing with solvents ensures a smooth distribution, so every square inch painted gains better weather resistance. Paints that shrug off rain cost less to upkeep and work harder under harsh conditions.
Every rainy week, glass treated with Octylmethyldimethoxysilane proves its worth. Wipers clear my windshield faster because water beads and streams away. Manufacturers apply it as a thin liquid layer over glass. On fabric or leather, a similar treatment turns porous fibers into moisture barriers. Carpets at home or upholstery in cars keep spills from soaking in and ruining materials.
Hotels seeking longer-lasting soft goods often treat curtains and cushions this way. Fewer stains mean less chemical scrubbing, and that pays off for businesses pinching pennies on laundry and replacements.
Everyday plastic parts—from window seals to phone cases—gain better resistance to grime and weather through this silane’s chemistry. Process engineers toss it into the mixing stage, pairing it up with other fillers or stabilizers. Once baked and cooled, these parts don’t crack as quickly, and their surfaces look cleaner, longer.
Shoes and outdoor gear with rubber soles also get an upgrade. By boosting water resistance in the rubber blend, boots keep feet dry longer and survive more puddle stomping.
Silane’s impact in construction isn’t subtle. When building with concrete or mortar, blending Octylmethyldimethoxysilane with water before mixing ensures that any finished surface stands up to freeze-thaw cycles and chemical attack. Old buildings crumble after decades of water sneaking into cracks, but this simple tweak slows that process down.
Adding silane poses no risk for workers when handled right, yet delivers a real difference in how long structures last. Fewer repairs reduce costs and protect both property and people.
Companies using this material can’t ignore health and safety. Strict safety data sheets spell out how to store, mix, and dispose, avoiding worker harm and protecting the environment. Trained hands measure out what’s needed, suit up with gloves and goggles, and ventilate their workspace. Labs and regulatory agencies continue to review long-term effects, checking for possible impacts across the supply chain.
Builders, manufacturers, and end-users all face the same pressures: fighting off mold, weather, and wear. Octylmethyldimethoxysilane offers a practical edge. Training and transparency go hand-in-hand with clever product design. When everyone in the supply chain shares honest data and encourages safe use, new chemistry paves the way for better, longer-lasting results in daily life.
Anyone storing chemicals in a lab or at a worksite knows the frustration of discovering a drum has gone bad before opening it. With Octylmethyldimethoxysilane, what sits on the label often misses the reality of storage conditions. The paperwork typically says 12 to 24 months under “optimal” storage. That promise assumes a dry, cool place, tightly sealed containers, and no sudden swings in temperature or humidity. Keeping these compounds away from moisture and acids stretches storage time—but few places outside a stockroom hit those marks perfectly. Contamination by water quickly kicks off hydrolysis, leaving behind silanols and a mixture that no longer performs as advertised.
Walking into a chemical storeroom, I always notice the containers that look a little sticky or misshapen. With Octylmethyldimethoxysilane, discoloration, increased viscosity, or any sign of cloudiness means the product faces hydrolysis or contamination. That may sound overly obvious, but busy techs sometimes chalk off odd appearance as batch variation. Once degraded, this silane loses effectiveness, especially in water repellence and adhesion-promoting roles. Testing for purity, like gas chromatography or FTIR, gives a straight answer but eats time and budget. Most facilities check the expiry date, peek at the contents, and run a trial batch.
Octylmethyldimethoxysilane turns up all over: concrete protection, paints, sealants, and even electronics. Its real power comes from forming durable bonds at surfaces, making water bead off or paint stick better. After shelf life passes, reliability drops fast. Engineers in building maintenance talk about failed coatings where water soaks through days after application. Bad silane wastes more time than it saves in cost, since teams get called back to refinish jobs. In electronics, degraded silane means poor insulation or corrosion protection—and nobody enjoys tracing failures on a circuit board.
In many labs, the storage area gets checked monthly. Labels display both the date received and an expected retirement day. Good practice means using smaller drums, so opened containers finish fast, reducing the odds of moisture entry. Some supply vendors now offer smaller pre-packed quantities at a slight premium, knowing waste costs more in the long run. Agencies like OSHA require up-to-date Safety Data Sheets on hand, making it easier for operators to catch shelf life warnings before use.
Digital inventory controls and barcode tracking help busy sites keep tabs on what's about to expire. Barcode apps on phones or tablets allow instant scanning and sign-offs, cutting forgotten inventory down to almost none. Annual or even quarterly chemical purges, though tedious, stop degraded silane from slipping into vital processes. Training new staff on why shelf life matters—sharing stories about ruined batches and callbacks—sticks better than any lecture. That hands-on memory shapes a more vigilant workplace, one where quality beats out corner-cutting every time.
Manufacturers may deepen efforts around tamper-evident seals, desiccant-packed containers, or even RFID chips for environmental tracking. Users have a role by keeping logs, flagging off-spec product faster, and not gambling on expired stock. Stronger teamwork between buyers, storage crews, and end users keeps Octylmethyldimethoxysilane effective—from the warehouse shelf to the application on site.
| Names | |
| Preferred IUPAC name | octyl(methyl)dimethoxysilane |
| Other names |
Dimethoxyoctylmethylsilane n-Octylmethyldimethoxysilane Methyloctyldimethoxysilane MDMO |
| Pronunciation | /ˌɒk.tɪlˌmɛθ.əl.daɪˌmɛθ.ɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 16415-12-6 |
| Beilstein Reference | 1361164 |
| ChEBI | CHEBI:88855 |
| ChEMBL | CHEMBL1907870 |
| ChemSpider | 187405 |
| DrugBank | DB11222 |
| ECHA InfoCard | 07b3e7c0-60be-4a88-9bda-adb5a333d404 |
| EC Number | 245-595-5 |
| Gmelin Reference | 1461640 |
| KEGG | C18606 |
| MeSH | D017180 |
| PubChem CID | 86745 |
| RTECS number | RW0150000 |
| UNII | I95E03PE79 |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID2050192 |
| Properties | |
| Chemical formula | C11H26O2Si |
| Molar mass | 234.43 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.88 g/cm3 |
| Solubility in water | Insoluble |
| log P | 2.9 |
| Vapor pressure | 1.3 hPa at 25 °C |
| Acidity (pKa) | 12.5 |
| Basicity (pKb) | >14 (pKb) |
| Refractive index (nD) | 1.415 |
| Viscosity | 1 mPa·s |
| Dipole moment | 1.05 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 510.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -381.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1613 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P310, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P405, P501 |
| Flash point | 77 °C |
| Autoignition temperature | 270 °C |
| Explosive limits | Lower: 0.6% ; Upper: 3.9% |
| Lethal dose or concentration | LD50 (Oral, Rat): > 2,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral Rat > 2000 mg/kg |
| NIOSH | GVG34860 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 3.5 mg/m3 |
| Related compounds | |
| Related compounds |
Trimethoxy(octyl)silane Octyltriethoxysilane n-Octyltrichlorosilane n-Octyltriacetoxysilane Methyldimethoxysilane n-Propyltrimethoxysilane |