
Chemists first synthesized organosilicon compounds like phenyltrichlorosilane at the turn of the 20th century. Back then, the focus was to exploit silicon’s unique bonds for new chemical pathways. Researchers at major European and American chemical companies began scaling up these reactions by the late 1920s. The actual push came during periods of industrialization and war, where there was a clear drive to find better materials for coatings, lubricants, and even insulation. Phenyltrichlorosilane’s structure allowed manufacturers to move away from brittle ceramics and fragile glass, offering more robust and versatile intermediates for specialty plastics and resins. Looking at patent trends, investment in this chemical stayed steady, reflecting its continued relevance as technology opened up more uses in microelectronics and synthetic chemistry.
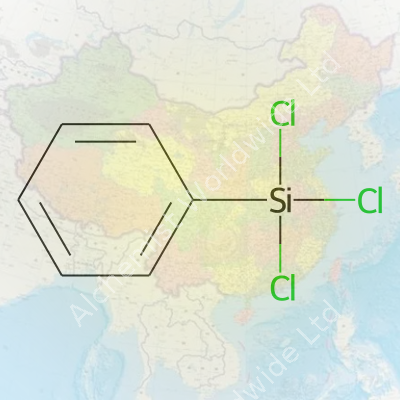
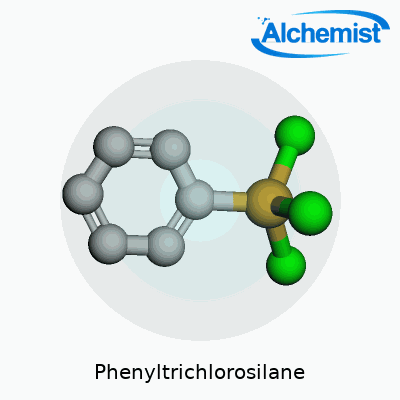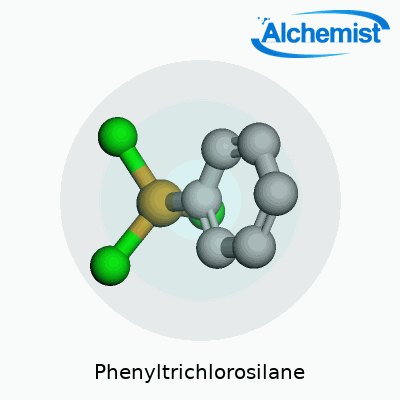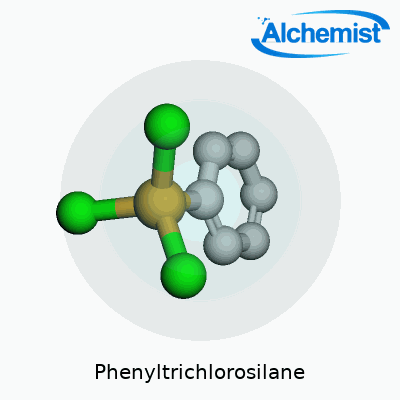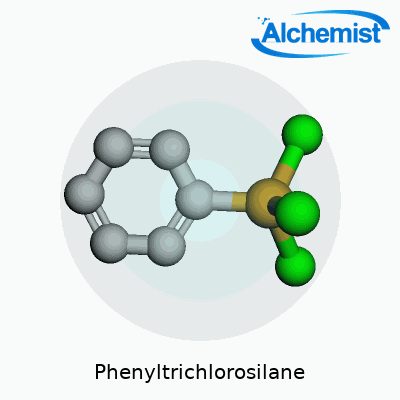
Phenyltrichlorosilane belongs to the chlorosilane family and features a phenyl group attached to a silicon atom, with three chlorine atoms bonded. It stands out for integrating organic and inorganic chemistry. Manufacturers prefer it where an aromatic group must be introduced onto a silicon-based backbone, often as a starting block for heat-resistant or hydrophobic materials. On the shelf, this chemical typically comes as a transparent to pale yellow liquid with a sharp odor—always packaged in tightly sealed glass or corrosion-resistant containers due to its volatility.
At room temperature, phenyltrichlorosilane remains a clear liquid. Its boiling point sits just over 195°C, and it shows a decent vapor pressure, meaning it can evaporate, posing risks during storage. The liquid density hovers around 1.3 g/cm³, denser than water and it does not mix with water, undergoing vigorous hydrolysis instead. It’s worth noting this hydrolysis is exothermic, churning out hydrochloric acid and siloxane derivatives. Its molecular formula, C6H5SiCl3, provides the backbone for many organosilicon architectures. Under UV exposure or heat, it can break down into various by-products, requiring careful storage away from light and moisture.
Suppliers give detailed specifications on container labels, reflecting industry standards for traceability and worker safety. Industrial batches achieve purities over 98%. Labels always call out the UN number (UN 2987), hazard pictograms for corrosives and toxins, and storage advice warning against contact with water. Regulatory paperwork, such as safety data sheets, accompanies every shipment, listing exposure limits, first aid steps, and spill-handling tips that comply with OSHA and REACH. Lot numbers trace back to individual synthesis batches, which provides accountability if anything goes awry downstream.
Industrial synthesis often relies on direct chlorination of phenylsilane, typically at elevated temperatures with catalysts to maximize yield. This process demands close control, as incomplete reactions or excess heat can lead to by-products that gum up production lines or threaten plant safety. Post-reaction, purification involves careful distillation under inert atmospheres, given the compound’s sensitivity to moisture. Handling waste from this process—loaded with hydrochloric acid and unreacted silanes—requires robust containment and scrubbing systems, or the result damages both equipment and the local environment.
Phenyltrichlorosilane holds a crucial place as both a reactant and an intermediate. Its three chlorine atoms are primed for substitution reactions, such as conversion with alcohols to form siloxane bonds or with amines for advanced polymers. Scientists routinely use it to graft phenyl groups onto silica surfaces, improving chemical resistance and surface properties. Its chemistry also enables crosslinking in silicone elastomers, driving development in sealants and thermal coatings. Experiments that tweak temperature, solvent, or catalyst often unearth new materials, broadening its use beyond older formulas of silicone rubber.
Traders and scientists recognize this molecule by names like trichlorophenylsilane, phenyl silicon trichloride, and Silres K3. Each name hints at specific applications. For companies selling to manufacturers, branding strategies use catalogue numbers, which link back to quality grades and packaging options. In research journals, shorthand names abound, but the key remains clarity—chemical accuracy helps ensure consistent procurement and safe handling.
Direct contact with phenyltrichlorosilane, even briefly, can scorch skin and eyes, while inhaled vapors irritate the airways and lungs. Hydrolysis releases hydrochloric acid fumes, so laboratories and production plants enforce strict containment, with fume hoods and full-face respirators. Emergency showers and eye-wash stations must be nearby. Standard operating procedures train workers in transfer techniques under inert gases, and spill-response gear sits ready for action. Regulators watch disposal practices closely, mandating neutralization of waste streams. Decades of mishaps drove home the lesson: shortcuts in safety lead to costly service shutdowns and health crises. Even now, periodic reviews of handling standards and upgrades to equipment prevent complacency.
Silicone polymer production pulls in much of the phenyltrichlorosilane supply. This compound’s ability to anchor aromatic rings onto silicon centers delivers materials with thermal stability and water repellency, core needs in sealants, electrical insulators, and non-stick coatings for cookware. It shows up behind the scenes in the making of specialty glasses where it tweaks refractive properties. Electronics manufacturers deploy it as a surface treatment, providing adhesion or hydrophobicity, which increases device longevity. The material shapes up in lab research as well, where it acts as a building block in developing monolayer films for sensors and microfluidics. Across each industry, the choice comes down to the distinct qualities the phenyl group brings—aromaticity links strength with chemical flexibility, a rare blend.
R&D teams constantly look for ways to upgrade product properties, lower costs, or reduce hazards. The last decade saw a push toward green chemistry, swapping out toxic catalysts and solvents during phenyltrichlorosilane synthesis. Researchers also built smarter delivery systems, allowing precise placement of phenyltrichlorosilane on nanostructured surfaces. In the electronics sector, scientists are exploring how ultrathin layers of this compound can serve as gate dielectrics and passivation barriers for next-generation chips. Patent filings reflect a steady drumbeat of improvements, signaling technical confidence that there’s plenty of life left in this well-known molecule if innovators keep stretching its reach.
Years of animal testing and accident reviews shaped today's knowledge about the risks of phenyltrichlorosilane. Acute exposure studies track lung, eye, and skin irritation. Long-term risks revolve more around chronic respiratory problems and potential sensitization to silane derivatives. So far, researchers have not flagged high carcinogenic risk for this compound itself, but they urge care given the toxic breakdown product, hydrochloric acid. Environmental studies watch the impact of run-off and accidental spills. Studies show that, under typical use patterns, strict engineering controls keep workers below exposure limits. Public health experts recommend regular medical surveillance for workers, to catch any emerging health patterns early. Regulators update safety guidance in response to the latest toxicology reviews, never assuming the story is finished.
Innovation never settles down in the chemicals sector. As the world leans into higher performance materials and electronics keep shrinking, phenyltrichlorosilane’s blend of organic and silicon chemistry catches the eye of startup labs and tech giants alike. Some researchers are steering toward greener, lower-impact syntheses that reduce both emissions and energy budgets. Others push for even tougher, more versatile silicon-based coatings that can stand up to harsh industrial environments or medical sterilization. Looking at regulatory moves, trends point toward even tighter safety standards, shaping everything from how the raw material gets delivered to how end-of-life products break down. No one counts phenyltrichlorosilane out of the future, and those with deep technical knowledge will set the pace for safer and more sustainable uses in the next wave of silicon science.
Phenyltrichlorosilane goes by the formula C6H5SiCl3. Folks working with silicone chemistry or advanced materials might run into this compound more than they notice. The chemical holds importance not just for what it is, but for what it allows manufacturers to do. I remember the first time I read its data sheet: so many caution notes, but also a world of possibility hidden in its reactivity.
Factories and labs use phenyltrichlorosilane as a core building block for silicone materials. The power of this molecule shows up in the way it helps splice organic groups onto silicon atoms. That reactivity lets chemical engineers build new molecules that survive both heat and harsh chemical environments, something most plastics shy away from. You find it in coatings, sealants, adhesives—places where you want materials to shrug off water, heat, or UV radiation.
Silicones grown from phenyltrichlorosilane turn up in protective coatings for electronics, taking on the job of keeping moisture out. This keeps circuit boards running in humid or damp conditions—think phones at the beach or industrial machines under harsh weather. Anyone who has opened up a piece of old electronics only to see the green, corroded tracks knows the pain moisture can bring. These coatings matter, and phenyltrichlorosilane sits at the beginning of their chemistry.
The big trick with phenyltrichlorosilane comes from those three chlorine atoms. By tweaking what sticks to the silicon, chemists can craft resins, gels, or harder materials for different demands. In the lab, the compound becomes a sort of Swiss Army knife—maybe you're working on water repellency, maybe you're aiming for thermal insulation. I've watched colleagues blend it with other ingredients to spin off specialty polymers, where even a small change in formula makes a huge difference in durability.
This flexibility brings another angle: tailored surfaces. Glass treated with phenyltrichlorosilane gains a water-beading property, letting rain slide off instead of causing ugly streaks. Ships use these sorts of coatings to cut back on saltwater corrosion and marine buildup. In optics, lenses coated with silicones prepared from this chemical stay clearer in damp conditions.
Handling phenyltrichlorosilane means respecting its hazards. The chlorine atoms make it reactive, alkylating, and, with water around, it fumes like crazy. I've seen a few gloves eaten up by careless splashes in university labs. Safety training for this chemical can’t just be a checkbox; it keeps people from getting burned or breathing toxic fumes. Industrial users gear up with respirators, face shields, and solid ventilation for good reason.
The production of phenyltrichlorosilane creates a need for responsible waste management. The chlorinated byproducts land on regulatory watchlists for air and water safety. Companies now invest in systems to scrub emissions and capture leftovers, aiming to balance industrial demand with the health of workers and neighborhoods. On the economic end, the compound supports jobs in chemical manufacturing and opens doors for exports, since its downstream products show up globally.
Some scientists are searching for milder processes, greener solvents, or recycling options for waste streams. Progress in this area could cut costs, sidestep supply snags, and take pressure off local environments. From what I’ve seen, change comes slowly, but improvements won’t stop as long as the risk, reward, and responsibility are clear.
Phenyltrichlorosilane carries the chemical formula C6H5SiCl3. That means it consists of one phenyl group (a six-carbon benzene ring, C6H5), attached to one silicon atom, which in turn holds on to three chlorine atoms. Simple on paper, but this combination packs a punch in the real world of chemistry and industry. Anyone who worked in a lab dealing with organosilicon chemistry probably remembers the smell and volatility of this liquid—and the care needed to handle it.
Within industry and research spaces, C6H5SiCl3 finds itself playing a variety of roles, acting as a building block for silicone-based polymers, coatings, and sealants. Those heat-resistant baking mats or flexible electronics don’t just appear—they rely on chemical players like this one. Because phenyltrichlorosilane contains both a reactive silicon center and a benzene ring, chemists use it in syntheses where both organic and inorganic worlds need to meet.
I can remember a senior colleague sharing tales of the early days at a coatings manufacturer, where phenyltrichlorosilane's ability to bridge these two worlds led to innovation in water-repellent fabrics. The real magic came from the formula’s flexibility: the three chlorine atoms are ready to react, typically getting swapped out for other groups that tailor the material’s performance.
Dealing with organosilanes requires more than lab smarts—it takes respect. Phenyltrichlorosilane reacts sharply with water, releasing hydrochloric acid and heat. A small spill quickly produces fumes that sting the eyes and nose. Negligence or improper handling can wound workers and pollute drains, so strict storage and disposal routines aren’t just bureaucratic hoop-jumping.
Chronic exposure to the vapors presents risks for workers. Regulatory agencies set workplace exposure limits for chlorosilanes, including phenyltrichlorosilane, and smart companies supply strong ventilation and robust personal protective gear. The industry needs to keep pushing for less hazardous alternatives or improved engineering controls. Investing in worker health programs—regular lung function checks, thorough safety training—pays off in fewer accidents and financial hits due to lost workdays.
The path to safer and more sustainable chemistry asks tough questions about compounds like C6H5SiCl3. Researchers keep looking for ways to replace harsh chlorosilane chemistry with alternatives that don’t churn out hydrogen chloride or hazardous byproducts. Greener upgrades and recycling technologies are growing, letting companies recover and reuse more silicon-based materials without generating as much waste.
Chemists in universities and industry labs alike work to harness the unique properties of the silicon-benzene pairing, finding new uses with less environmental toll. Funding and open information sharing help power innovation—and anyone who has navigated government grant writing or patent filings knows just how valuable collaborative progress feels.
C6H5SiCl3’s footprint stretches across the materials that shape modern living. Science thrives on formulas, but no molecule exists in a vacuum. With a clear chemical formula and a track record of practical uses, phenyltrichlorosilane shows that every building block brings responsibility along with versatility. Genuine progress in chemistry means finding smarter ways to use such tools, keeping people and the world safer along the way.
Phenyltrichlorosilane isn’t a name you run into at the grocery store. It’s a chemical that can get nasty fast, especially when it meets water or even humid air. If you take off the cap and let it mix with moisture, clouds of hydrochloric acid gas start billowing up. That’s both a breathing hazard and a skin risk, even for chemists who spend long hours in the lab. I remember my first encounter with it—just a few stray drops near a wet bench sent everyone scrambling for the vent hood and goggles. So, respecting its reaction habits isn’t just about rules, it’s common sense born from experience.
No one should handle phenyltrichlorosilane without proper gear. Nitrile or butyl rubber gloves always stay between my skin and the bottle. Regular latex gloves break down too easily. Safety goggles never come off, and I add a face shield for large pours or transfers. Lab coats fit snugly, sleeves fully covering arms, and long pants and closed shoes turn into must-haves. I still shake my head remembering a time a new intern skipped gloves “just for a second”—that “quick” moment ended with angry red welts and a lesson he never forgot.
Good airflow is your friend. Fume hoods aren’t just fancy furniture—they pull dangerous fumes away before you breathe them. Before starting, I always double-check that the sash drops easily and that airflow monitors are in the green. Spills and splashes can ruin more than your workday, so lining benches with absorbent pads gives you a first line of defense. Emergency showers and eyewash stations sit within reach, with nothing blocking the path. I make a habit of walking new folks around to show these spots—because you only look for them in a hurry when it’s already too late.
Phenyltrichlorosilane needs dry, cool storage, far from any water sources. One careless move with a leaky faucet nearby, and you could have an acid cloud. Tight caps, proper labeling, and sturdy secondary containers matter; I’ve seen plastic bins stop a bad leak from wrecking an entire cabinet. Storage logs look tedious until you go searching for a missing bottle, so accurate record-keeping saves time and prevents scary surprises.
Spills freak everyone out, but panic’s the enemy. Sand or vermiculite soaks up liquids and lets you scoop them safely into a proper waste container. Dumping it down the drain never works out—every old-timer has a horror story about clogged pipes and chemical burns. Disposal goes through licensed hazmat crews, not the regular trash. One guy who learned this the hard way still handles unwanted bottles with white-knuckled care to this day.
Safety drills and proper training keep everyone sharp. Inexperienced users benefit from hands-on demos, showing exactly how a runaway reaction looks and how to respond. Regular team meetings sniff out sloppy habits before they turn into accidents. I’ve found that open conversations, not scolding, encourage everyone to speak up about near-misses and forgotten steps. Business as usual in the lab stays safe because people rely on experience, not luck.
Phenyltrichlorosilane doesn’t forgive careless mistakes. Over the years, I’ve worked in labs where just a minor slip-up with a reactive compound like this one would set off the fumes, the fire alarm, or worse. Any chemist who’s opened a bottle knows: touch of water, you get hydrochloric acid gas, which will burn your lungs and eyes. So it’s not just about following rules on a page—real life safety means thinking through your storage every step of the way.
Chlorosilanes, including phenyltrichlorosilane, deliver major value in semiconductor manufacturing, lab research, and specialty coatings. Health experts set strict limits for exposure. If someone inhales even a small whiff, they risk choking, coughing, corrosive injury. Spillage onto skin means pain, possible hospitalization. Messes, leaks, or pressure build-up in a hot storeroom can trigger a disaster. Companies like Dow Corning and Wacker Chemie often advise in their safety sheets—keep the container sealed tight, store cool, dry, well-ventilated, far from water sources.
I’ve seen what happens if basics get overlooked. Someone leaves the bottle near a sink—days later, the cap pops right off, and everyone’s running for the eyewash station. So, store the bottles in stainless steel cabinets lined with dry absorbent. Put them on shelves with wide lips to catch drips. Never place them above eye-level. Each workplace has safety data sheets on file, but real-world handling means double-checking for leaks, inspecting every week, keeping incompatibles—like water, bases, or even damp rags—locked away on another shelf.
Labs get hot, especially late in summer or with everyone working after hours. Don’t count on air conditioning to save the day. Chlorosilanes break down faster in heat, so keep the storeroom below 25°C—my own experience says that insulation or a dedicated cooling unit pays off long term. Fresh airflow counts for more than most folks think. Not a fan blowing fumes around, but a vent hood, full exhaust, negative pressure if possible. Corrosive vapors never go easy on copper pipes or ceiling fixtures, so plastic or stainless ducting lasts longer.
Even the best storage fails sometimes. Every facility should keep spill kits nearby—neutralizer for acids, chemical-absorbent pads, tightly lidded waste containers. Everyone working in storage wears splash goggles, gloves, full aprons. Monthly drills matter. I’ve witnessed too many workers panic when an alarm rings, just because it had been a year since their last walkthrough. Make eye wash and shower stalls easy to reach with zero clutter or blocked doors.
Trust forms over time, not just from training, but from a culture of asking questions and reviewing storage logs. It helps to set up a simple checklist: check temperature, look for corrosion on shelves, test the alarm system, count up PPE gear. Some labs invest in smart sensors for vapor detection. Even on tight budgets, refusing to cut corners on safety keeps everyone in the building a little more relaxed and a lot more productive.
Phenyltrichlorosilane brings a lot to the table thanks to a couple of things you notice straight off: it looks like a clear, colorless liquid and lets off a sharp, stinging odor if spilled or left open. If you get some on your hands or in your eyes, it burns—nobody forgets that in the lab. The chemical formula, C6H5SiCl3, tells you it links a phenyl group (that’s your basic benzene ring) to a silicon atom loaded with three chlorine atoms.
Its boiling point settles right under 200°C, but it only takes some warmth to help the fumes rise. In a closed bottle, even room temperature sends vapor into the air. The liquid isn’t thick; it runs across a glass plate almost as easily as water. If moisture hangs around, phenyltrichlorosilane starts reacting, releasing hydrochloric acid gas—your eyes and nose will notice that fast.
Mixing phenyltrichlorosilane with water happens quickly. The chlorine atoms jump ship, and hydrogen chloride fumes up. What’s left behind gels up into siloxane polymers, the sort of backbone you find in high-tech coatings or sealants. It’s also pretty eager to bond with alcohols, shooting out more hydrochloric acid and building structures chemists rely on in all sorts of silicone products. The process can go wrong if you rush or skip the right gear—gloves, goggles, and a fume hood aren’t optional.
Bringing it near bases or strong oxidizers turns risky. It doesn’t take much for its vapors to irritate airways or skin, either. Proper ventilation matters in any space using it, and everyone involved learns to treat spills like emergencies. Once you see glass or metal equipment corrode near the stuff, you understand why safe storage uses special materials.
Phenyltrichlorosilane’s edge comes from its ability to build up networks with both organic and inorganic partners. In electronics, it helps craft heat-resistant coatings and water-repelling layers on microchips. Surface treatment of glass or metals becomes straightforward with it, making materials last longer or pick up new chemical personalities.
Nobody can ignore the downsides. It’s toxic, even at low concentrations, pushing manufacturers to double-check their ventilation and spill protocols. I remember coworkers rushing to fix a tiny leak—I still picture how quickly the sharp, acidic odor filled the air, sending us scrambling for the exit and safety gear. The risk nudges labs and factories to use automated mixing and remote monitoring.
Research keeps searching for ways to handle phenyltrichlorosilane without putting people in the line of fire. Engineers look into closed systems that seal off fumes and reduce direct handling, which means less exposure. Training matters just as much—knowing the properties makes everyone take precautions seriously, even if it slows the process down.
Some teams examine new silane compounds with fewer hazards, hoping for similar technical results. Old-fashioned drip additions and open glassware rarely show up in places that work with any trichlorosilane; you learn to respect the substance and expect the unexpected. For now, phenyltrichlorosilane still anchors specialty silicone chemistry, but nobody should overlook the need for constant vigilance.
| Names | |
| Preferred IUPAC name | Trichloro(phenyl)silane |
| Other names |
Trichlorophenylsilane Phenylsilicon trichloride Silane, trichlorophenyl- Trichloro(phenyl)silane |
| Pronunciation | /ˌfiːnaɪl.traɪˌklɔːr.oʊˈsaɪleɪn/ |
| Identifiers | |
| CAS Number | [98-13-5] |
| 3D model (JSmol) | `Phenyltrichlorosilane JSmol 3D model string: "CC1=CC=CC=C1[Si](Cl)(Cl)Cl"` |
| Beilstein Reference | 1209242 |
| ChEBI | CHEBI:52068 |
| ChEMBL | CHEMBL153480 |
| ChemSpider | 21502 |
| DrugBank | DB11225 |
| ECHA InfoCard | 100.006.021 |
| EC Number | 203-852-3 |
| Gmelin Reference | Gmelin 54295 |
| KEGG | C18994 |
| MeSH | D010629 |
| PubChem CID | 66205 |
| RTECS number | TC6300000 |
| UNII | 8Q85271U4S |
| UN number | UN1810 |
| Properties | |
| Chemical formula | C6H5SiCl3 |
| Molar mass | 243.57 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Pungent |
| Density | 1.38 g/mL at 25 °C (lit.) |
| Solubility in water | Reacts |
| log P | 2.9 |
| Vapor pressure | 1 mmHg (25°C) |
| Acidity (pKa) | Acidity (pKa): -3.3 |
| Basicity (pKb) | 3.5 |
| Magnetic susceptibility (χ) | -73.0e-6 cm³/mol |
| Refractive index (nD) | 1.518 |
| Viscosity | 4 cP (25°C) |
| Dipole moment | 1.00 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 340.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -369.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -933.7 kJ/mol |
| Pharmacology | |
| ATC code | V09CX04 |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, reacts violently with water, releases toxic and corrosive gases (hydrogen chloride and silicon oxides), harmful if inhaled. |
| GHS labelling | GHS02, GHS05, GHS06 |
| Pictograms | GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H301, H311, H314, H331 |
| Precautionary statements | H261, H301, H314, H331, P210, P260, P264, P270, P271, P280, P301+P310, P303+P361+P353, P304+P340, P305+P351+P338, P311, P330, P363, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 3-2-0-W |
| Flash point | 72 °C (closed cup) |
| Autoignition temperature | 220 °C |
| Lethal dose or concentration | LD50 oral rat 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 1800 mg/kg |
| NIOSH | TL6825000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Phenyltrichlorosilane: 5 ppm (parts per million) |
| REL (Recommended) | 1.5 to 3 ppm |
| IDLH (Immediate danger) | 50 ppm |
| Related compounds | |
| Related compounds |
Phenyldichlorosilane Phenylsilane Trimethylsilyl chloride |