
Long before today’s specialty chemicals industry turned to tailored silanes, chemists in the twentieth century started shaping silane chemistry for tougher coatings, resilient adhesives, and high-performance sealants. Phenyltriethoxysilane emerged from early organosilicon research, fueled by the need for more durable, moisture-resistant materials in electronics and construction. Researchers noticed the powerful effect of silicon-oxygen bonds and organic functional groups, leading to the synthesis of organofunctional silanes, such as phenyltriethoxysilane. As companies built up expertise in the post-war era, enthusiasm for phenyl derivatives grew, with phenyltriethoxysilane joining the toolbox for crosslinking, adhesion promotion, and surface treatment technologies. The compound gained ground as new industries demanded tougher polymers and lightweight composites. By the turn of the millennium, labs around the world used this silane to anchor organic chemistry onto the backbone of glass, metal, and ceramic surfaces.
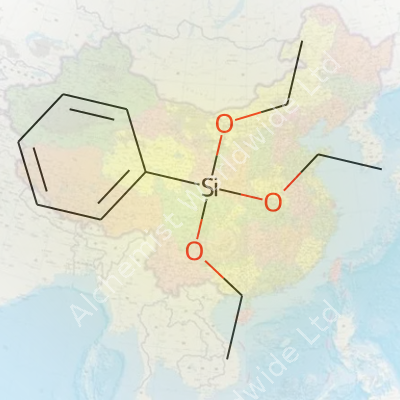
Phenyltriethoxysilane stands out due to its hybrid structure: a phenyl group paired with silicon, capped by three ethoxy groups. This unique makeup helps bridge organic and inorganic materials. The ethoxy groups react with water or hydroxylated surfaces, making phenyltriethoxysilane a popular choice for coupling agents in high-purity glass reinforcement and specialty polymer synthesis. It forms strong, moisture-stable bonds and imparts hydrophobic properties—crucial for applications like optics, insulation, and electrical encapsulants.
A typical sample of phenyltriethoxysilane appears as a clear, colorless to pale yellow liquid with a sharp, almost pungent odor, a result of the ethoxy groups. It has a molecular formula of C12H20O3Si and a molar mass around 240.37 g/mol. Its boiling point sits close to 240–245°C, making it handy for processes requiring heat stability. Density hovers near 1.0 g/cm³ at room temperature. The compound dissolves well in common organic solvents like toluene, ethanol, and ether, but reacts when water enters the picture—the ethoxy groups hydrolyze, setting the stage for siloxane bonding.
Producers generally package phenyltriethoxysilane in airtight, amber glass or lined steel containers, labeled with hazard warnings due to flammability and possible skin irritation. Detailed labeling lists the molecular formula, purity (generally above 98%), moisture content, appearance, and lots of shipping data. Anyone handling this molecule needs compatible gloves (usually nitrile), splash-resistant goggles, and fume hoods to avoid vapor exposure. Companies often provide certificates of analysis guaranteeing low water content, essential for downstream silicon chemistry.
Manufacturers typically access phenyltriethoxysilane by reacting phenyltrichlorosilane with ethanol in the presence of an acid scavenger, often carried out under reflux conditions. This causes ethoxy exchange, swapping the chlorine atoms with ethoxy groups. The process requires meticulous control of moisture and temperature. Traces of water in the reaction will start hydrolysis early, which hampers yields. After the reaction, distillation purifies the product, stripping away ethanol and generating a compound with robust purity, fit for demanding technical markets.
The starring feature of phenyltriethoxysilane lies in those ethoxy groups. They hydrolyze in the presence of water and acid or base catalysts, replacing ethoxy with hydroxyl. The newly formed reactive silanols then condense with surfaces or other silanols to form rigid Si–O–Si frameworks—this is silanization in a nutshell. Adding phenyl functionality gives treated materials thermal stability, chemical resistance, and unique wetting properties. Surface modification with phenyltriethoxysilane transforms coarse fillers into components for specialty rubbers or high-voltage electrical insulation. Chemists can incorporate other functionalities using downstream reactions on the phenyl ring, expanding potential use cases for advanced composites and hybrid electronics.
This compound shows up under several names, including triethoxyphenylsilane, phenyltriethoxysilane, and, in some catalogs, silane, triethoxyphenyl-. Organizations like the Chemical Abstracts Service (CAS) assign the identifier 780-69-8, clarifying sourcing and regulatory reporting. Manufacturers develop unique trade names, reflecting proprietary purity or minor formulation tweaks, but the chemistry stays consistent.
Handling phenyltriethoxysilane demands real discipline. The liquid forms flammable vapors; static sparks, open flames, or careless handling spark fires. Prolonged skin contact or inhaling vapors irritates mucous membranes, with risk rising in hot or poorly ventilated conditions. Companies need robust spill protocols and chemical-resistant gear. Workers should know the nearest eyewash station and safety shower location. Proper ventilation remains non-negotiable; local exhaust hoods or gloveboxes cut vapor risk and keep exposures below occupational limits. Storage guidelines call for tightly sealed containers, kept away from heat, acids, and bases.
Engineers rely on phenyltriethoxysilane for advanced composites, water-repellent treatment, and primer coatings. In glass fiber manufacturing, this silane’s ability to couple resin and glass transforms mechanical performance, giving lightweight structures new horizons in aerospace and automotive. Surface modification projects use phenyltriethoxysilane in semiconductor fabrication, optics, and anti-corrosion coatings. It finds other uses in paint additives, adhesives, and as an intermediate in specialty silicone release coatings. The phenyl group’s aromatic character tunes thermal resilience, making it a favorite in products exposed to unpredictable environments or prolonged heating.
Research on phenyltriethoxysilane continues in both academic and corporate circles. Scientists track the molecule’s surface chemistry using techniques like atomic force microscopy and solid-state NMR, trying to push the silane’s boundaries for abrasion, UV stability, and chemical inertness. Polymer chemists experiment with copolymerization using this silane, chasing stronger interfacial adhesion or new fire-retardant properties. Nanotechnology labs use phenyltriethoxysilane for thin film formation on silicon wafers, which matters for MEMS sensors and nanoelectronics. Studies explore tweaking the phenyl ring, adding substituents to unlock next-generation silane performance in specialty fields like biocompatible coatings or optoelectronics.
Animal and cell culture studies show vapor and liquid exposures can irritate tissues, especially eyes, skin, and lungs. Chronic exposure may sensitize skin or trigger respiratory symptoms, so occupational health officers conduct robust risk assessments. No strong evidence links phenyltriethoxysilane to mutagenicity or carcinogenicity at typical workplace concentrations, but long-term studies stay ongoing—especially as new downstream uses keep appearing. Monitoring workplace vapor levels, training workers, and rotating tasks help cut cumulative risks. Disposal requires full neutralization and care around hydrolysis byproducts, as ethanol vapors and acidic residues can create more hazards.
Industrial priorities drive more sustainable manufacturing of organosilanes like phenyltriethoxysilane. Green chemistry approaches—solvent recycling, safer precursors, and energy-efficient syntheses—signal where the industry goes next. Researchers aim to push phenyltriethoxysilane’s compatibility with renewable fillers and recycled substrates. In electronics, tighter control of interface chemistry means this silane could anchor next-gen flexible chips and bio-integrated sensors. As high-performance polymers grow common in infrastructure, phenyltriethoxysilane’s blend of organic and inorganic reactivity will set the pace for hybrid materials. Regulatory shifts and consumer concern with chemical exposure will keep manufacturers vigilant, demanding clear labeling, lower-toxicity protocols, and transparent toxicity research.
You won’t find phenyltriethoxysilane on a grocery store shelf, but much of what people use daily benefits from it. In chemical terms, it’s a silane coupling agent, which means it can connect two very different materials together. Picture a world without paint that sticks to glass, or electrical circuits that last under tough weather; that’s the world you’d have without chemicals like this one.
Glass doesn’t really get along with rubber or plastics on its own. Chemists rely on phenyltriethoxysilane to help forge these friendships. It adds to the adhesion between glass fibers and plastic resins to make sturdy fiberglass composites. Car bumpers, windmill blades, boats, and bathtub panels draw on this improved toughness. The reason this works—this compound latches onto silica on one end and carbon-based polymers on the other, almost like a handshake between two people from very different worlds.
Weather and moisture do real damage to microelectronics and coated surfaces. When engineers coat surfaces with phenyltriethoxysilane, water can’t do its usual mischief. This ability comes in handy when electronics must last for years, even outdoors or in bathrooms. A water-resistant barrier forms as the silane creates a thin but durable layer. I’ve seen manufacturers skip this step, and it’s not long before devices break down or paint starts to flake. It’s easy to overlook details like this—until the failure happens.
Companies that make paints add phenyltriethoxysilane to keep coatings from peeling or losing their shine. The molecule helps coatings anchor themselves onto glass, ceramics, or metal surfaces. That’s important for big city buildings with huge glass windows. Without help from silanes, rain would eat away at paint jobs and make window cleaning a nightmare. Sometimes, these barriers even make surfaces easier to clean, cutting out harsh scrubbing and strong chemical cleaners.
Solar panel makers face constant challenges; panels should last years through storms, strong sun, and haze. A silane-based layer on the glass in solar panels keeps them clear and resistant to dirt and water droplets. Circuit boards in electric vehicles and advanced phones also use this chemistry. Tiny copper lines in a chip can corrode and fail without that layer of protection.
Working with phenyltriethoxysilane takes careful handling. It can release ethanol when it reacts with water, and both fumes and liquids need airtight facilities and protective gear. Regulations keep the worst risks in check, but constant vigilance from people in the labs and factories matters most. Over the years, stricter workplace monitoring has made a real impact, as I remember older stories of chemical burns or damaged equipment. Nothing beats hands-on safety routines and up-to-date equipment.
Some in the field now look at greener alternatives and ways to recycle waste from silane processing. My experience tells me, small changes in how developers synthesize, use, or recover silanes add up to big reductions in chemical waste. Moving forward, companies who invest in better waste handling and research not only help the environment but also cut long-term costs and boost reliability for end users.
Phenyltriethoxysilane shows up across many chem labs and industrial settings. Before digging into storage practices, it’s always smart to think about what goes wrong if you get careless. I’ve seen more than a few expensive headaches caused by simple lapses—like putting a reactive substance near a window or skipping a check on the drum’s seal. This compound reacts with water, forming corrosive byproducts and generating heat. That makes good storage not just a formality—a must for safety and the wallet.
Open-air exposure turns phenyltriethoxysilane into a leaky mess. Even a splash of humidity starts hydrolysis, which can ruin both the chemical and anything nearby. This isn’t just theory; I watched a colleague lose half a drum when a seal failed in an old warehouse over summer break. After that, I’ve always stressed keeping containers tightly closed in spaces with dry, filtered air. Desiccators or well-maintained dry cabinets perform best.
Cool, stable storage slows down unwanted reactions and makes spills less risky. Between 2°C and 8°C works for small bottles in a research setting, although large industrial stock often lands in climate-controlled storage rooms a little above refrigeration. Direct sunlight speeds up breakdown and pressure build-up. A friend’s warehouse ran into trouble during an August heatwave. He found three bulging containers by the end of the month—each one at risk of popping a cap or worse. Keeping things out of sunlight and well away from heat sources matters.
There’s another issue I learned the hard way early on. Containers and shelves touching strong acids or basic metals make trouble. The silicon in phenyltriethoxysilane reacts aggressively. Stainless steel works if you can’t use glass, but clear labeling helps workers avoid stacking things in a way that risks spills or cross-contamination. Some facilities use secondary containment bins—usually plastic or glass fiber—just to be sure.
Accidents happen, even when everyone follows rules. I saw an emergency handled much better thanks to clear Dating, hazard labeling, and up-to-date Material Safety Data Sheets close by. If someone needed to check first-aid instructions or alert the HazMat team, everything was laid out. Regular audits help, too. Chemical management teams benefit by catching expired or compromised stock before it causes surprise leaks or exposure.
Even with airtight containers, small vapors escape over time. Well-ventilated rooms keep fumes from creeping above safe limits. Fume hoods and exhaust fans take care of the heavy lifting. For spill response, stations with absorbent materials rated for silicon compounds and gloves suited for organic solvents make cleanup faster and safer.
Putting all of this into everyday practice beats learning from mistakes. I’ve always kept checklists and done routine walk-throughs, often finding shortcuts that may look fine but come back to bite later. Training everyone who might handle phenyltriethoxysilane, even if it’s just once a year, keeps mistakes rare and costly incidents off the list.
Many folks ask whether phenyltriethoxysilane is something you want near your home, your workshop, or your day job. Industry finds value in this silane as a surface modifier and coupling agent. It connects organic and inorganic materials in paint, plastics, adhesives, and electronics. But popularity does not mean it’s safe for freewheeling use. I’ve handled silanes in the lab myself, and I can say the safest approach is direct and informed respect.
Phenyltriethoxysilane is clear and has a sharp, distinctive smell. It's a liquid at room temperature, does not mix well with water, and has a relatively low boiling point compared to heavier silanes. That gives it a higher chance to vaporize, especially in a warm or poorly ventilated space. Silanes often bring certain risks into the picture — flammability, skin irritation, and damage to your eyes and airway. That’s not just a guess; it aligns with material safety data sheets and scientific research. The ethoxy groups in this molecule break down into ethanol and related compounds, both of which can irritate and can intensify fire hazards.
Short answer: It can pose problems and should not be handled carelessly. Skin contact brings a stinging, burning sensation, sometimes leaving a rash or blisters if not washed off. Eye exposure hits even harder; splashes can burn, possibly causing permanent injury. Breathing in the vapor may irritate your airways, leading to cough, sore throat, or worse symptoms at higher exposures. I remember the cautionary tales from lab techs who forgot their goggles or gloves even once. Reports show that chronic exposure — not just accidents — leads to headache, dizziness, and fatigue. Animal studies have hinted at more severe internal effects, but human data sticks mostly to acute irritation.
Fire risk isn’t something to brush off. Liquid phenyltriethoxysilane burns, releasing gases that harm lungs and eyes. In confined spaces, a spark spells real danger. No one wants to learn that lesson the hard way.
This substance falls under the “handle with care” umbrella. Factories take it seriously with storage protocols, ventilation systems, and medical first-aid plans. But students, tinkerers, and small business workers see it, too. A growing number of DIY electronics and restoration projects use silanes. Online shopping puts these bottles in more households. Curiosity shouldn’t end in a health scare. Simple gear like gloves, goggles, and airflow make a world of difference — and it’s not just a rule for big chemical companies.
European regulatory programs like REACH, and the U.S. OSHA Hazard Communication Standard, classify phenyltriethoxysilane as hazardous. The Globally Harmonized System for labeling chemicals gives it exclamation mark and flame icons for good reason. That puts responsibility on the user, not just the supplier.
If you must work with phenyltriethoxysilane, get a good fume hood or at least open windows wide. Wear goggles and gloves every time. Store the container tightly sealed in a cool, dry spot away from heat or sparks. Clean up spills fast and dispose of waste using local hazardous collection guidelines. Keep it away from food, pets, and kids. If you get any on your skin or eyes, rinse like your health depends on it — because it does.
Every chemical brings its own baggage. Phenyltriethoxysilane proves the point: useful in the right hands, risky without the right mindset and protections. Don’t treat it like just another bottle in the cupboard. Take the time to learn the facts, gear up accordingly, and look out for each other — whether you’re in a chemistry classroom or a garage workshop.
Many people jump straight to technical guidelines when dealing with chemicals like Phenyltriethoxysilane, but it's easy to miss just how dangerous simple negligence can be. From my own days working in labs filled with all kinds of reagents, I’ve seen firsthand how the smallest mistakes add up. The worst accidents I ever saw always started with a skipped glove, an unlabeled bottle, or a quick dismissal of a strong smell in the air. Phenyltriethoxysilane brings its own risks, and there’s no shortcut around keeping yourself and others safe.
This compound doesn't wait for you to be careless. A splash or a heavy vapor cloud can spread faster than you expect. Phenyltriethoxysilane burns the skin and eyes, and just breathing in its vapors for a moment can mess with your respiratory system. OSHA and NIOSH materials on similar alkoxysilanes list constant glove use and eye protection as non-negotiables. Real-world experience agrees: Your standard-issue nitrile gloves work, but you’ve got to swap them out as soon as you notice any weakness or swelling, since this stuff eats through worn-out gear.
Forget about rolled-up sleeves and thin disposable gloves. In settings I’ve worked in, we used chemical splash goggles, heavy-duty nitrile or butyl gloves, and lab coats with sleeves fully down. Anyone dealing with larger volumes switched to a face shield and used a full chemical apron. Lax dress standards don't just break the rules—they set up opportunities for accidents you don’t walk away from easily. Think about how your workplace sets up for splash risk, not just spills on the bench. Closed shoes, not sandals, keep feet clear of danger. These small decisions make long days in the lab something you can survive without scars or stories you wish you didn’t have.
In every industrial space I’ve been in, proper ventilation isn't about ticking a box. It shapes whether colleagues end up complaining about headaches or coughing in the break room. Phenyltriethoxysilane vaporizes faster than you expect, especially during pouring and mixing. Fume hoods pull those vapors away before you ever realize you forgot to tighten a cap. Good ventilation cuts down on long-term health problems—cases of chronic allergies and breathing issues come up way less in shops that take this seriously.
It's easy to see bottles shoved behind boxes, far from safety showers or eye wash stations, in rushed labs or warehouses. Safe handling grows out of smart storage: keep sealed containers in cool, dry spots, far from sources of water or acids. Water and Phenyltriethoxysilane create heat and dangerous byproducts. In my experience, putting emergency numbers and procedures front and center—right by the entryway or main worktable—actually keeps things clearheaded if something goes wrong. You won't remember a complicated flowchart if your eyes sting and you’re racing for clean air.
What I’ve learned after years in chemical environments is that proper handling doesn't rest just on a single safety manual. It grows from repeated practice and open discussion. Bring up close calls at shift meetings. Update posters and training briefings with recent incidents and new safety steps. Foster a space where people point out unsafe practices—not just their own mistakes. Mutual care, more than any single glove or eyewash bottle, keeps real disasters at bay.
Working around chemical storerooms, you learn to respect the difference between something written on a label and what happens in practice. Phenyltriethoxysilane, a clear liquid used for surface treatment and silane coupling, often shows a labeled shelf life of about a year in tightly sealed original containers. That’s a manufacturer’s suggestion built around steady, ideal storage. In my experience, common mistakes often cut into that time window, or spoil the whole batch even sooner.
Behind every date stamped on a container lies a set of assumptions. Manufacturers base this estimate on pure samples, carefully sealed, left in dry storage at about 20–25°C, protected from sunlight and oxygen. These conditions limit condensation, hydrolysis, or other unwanted reactions. Out in the field, chemical containers rarely see that kind of pampered life. Temperature swings in a warehouse, half-sealed caps, and the occasional “just grab a little more” all challenge the shelf life number.
Moisture is trouble for phenyltriethoxysilane. Once water creeps in, hydrolysis kicks off. That breaks down the silane, causes cloudiness, possibly some precipitate, and the surface chemistry goes out the window. If the stuff smells sharper or looks milky, that’s your sign: something’s gone wrong. Heat accelerates decomposition. I saw a batch kept in a shipping container during a summer week change color—no trick of the light, just a clear sign of heat damage.
A failed batch doesn’t just waste dollars. Projects using degraded silane often see adhesion problems, failed coatings, or wasted labor—problems that spiral. Responsible chemists test for purity. In my old lab, we checked the refractive index and ran a small-scale application test. If the silane failed that round, it went straight to the disposal drum. Scrimping here often means bigger losses down the line.
Using secondary containers cuts down accidental moisture exposure. Never pour unused material back into the main drum. Always check the expiration date, but pay attention to what you see and smell. If a container sat open in a humid area—even for a few minutes—write the date on the drum and plan for quicker use. Electronic inventory systems help flag materials close to recommended shelf life, and rotating inventory (first-in, first-out) stops old stock from festering unnoticed on the far shelf.
A little discipline around storage and handling stretches that shelf life much closer to the manufacturer’s estimate. Train your crew, invest in better seals, and keep records on storage conditions. Newer drums with built-in desiccant caps add a safety margin. Every lab and warehouse faces the same chores—push for real accountability, not excuses. If you rely on phenyltriethoxysilane, your best defense against chemistry gone wrong starts long before you pop the first seal.
| Names | |
| Preferred IUPAC name | triethoxy(phenyl)silane |
| Other names |
Triethoxyphenylsilane Phenylsilane, triethoxy- Silane, triethoxyphenyl- |
| Pronunciation | /ˌfiːnaɪlˌtraɪˌɛθɒksiˈsaɪleɪn/ |
| Identifiers | |
| CAS Number | 780-69-8 |
| Beilstein Reference | 1362200 |
| ChEBI | CHEBI:40790 |
| ChEMBL | CHEMBL16264 |
| ChemSpider | 186463 |
| DrugBank | DB11299 |
| ECHA InfoCard | 03e91500-0000-4e67-87d0-1a89005e7f59 |
| EC Number | 202-924-1 |
| Gmelin Reference | 88848 |
| KEGG | C19102 |
| MeSH | D010624 |
| PubChem CID | 66230 |
| RTECS number | VV8400000 |
| UNII | VL3WLK5Z2C |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C12H20O3Si |
| Molar mass | 318.48 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Aromatic |
| Density | 0.970 g/mL at 25 °C(lit.) |
| Solubility in water | Insoluble |
| log P | 2.6 |
| Vapor pressure | 0.7 hPa (at 20 °C) |
| Acidity (pKa) | 10.6 |
| Basicity (pKb) | −3.6 |
| Magnetic susceptibility (χ) | -64.0e-6 cm³/mol |
| Refractive index (nD) | 1.436 |
| Viscosity | 2.5 mPa·s (25 °C) |
| Dipole moment | 3.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 489.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3687 kJ mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H302, H319, H332 |
| Precautionary statements | Precautionary statements: "P210, P233, P240, P241, P242, P243, P260, P264, P271, P280, P301+P310, P303+P361+P353, P304+P340, P305+P351+P338, P312, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0-ان |
| Flash point | Flash point: 57 °C |
| Autoignition temperature | 370 °C |
| Explosive limits | 1.2% (LEL), 10.5% (UEL) |
| Lethal dose or concentration | LD50 (Oral - rat) 3600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat (oral): 7.12 g/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1-1.0 ppm |
| Related compounds | |
| Related compounds |
Phenyltrimethoxysilane Methyltriethoxysilane Vinyltriethoxysilane Propyltriethoxysilane n-Butyltriethoxysilane Phenyltrichlorosilane |