
Chemists first started working on phenyltrialkoxysilanes in the early days of organosilicon chemistry, right after World War II. Back then, research grew arms and legs as efforts aimed to build better materials for everything from aircraft to paint. Phenyltrimethoxysilane came up as a specialty because its silicon-carbon bond offered a handle to link organic molecules to glass or metal surfaces. The surge of interest in plastics and composite materials through the 1960s cemented its spot as a handy tool. Today, a lot of the advances we see in everything from waterproofing compounds to electronic coatings can be traced back to these early discoveries and refinements in silane chemistry. Generations of lab workers have tweaked methods, shifted solvents, and streamlined yields to make phenyltrimethoxysilane both easier and safer to produce.
Phenyltrimethoxysilane falls into that niche group of chemicals that make things stick—literally. Its molecular structure, with a phenyl group bonded to silicon and three methoxy groups, gives it the versatility to anchor organic layers onto inorganic surfaces. In plain English, think of it as the bridge that helps paint grip glass or that allows flexible coatings to survive on hard ceramics. The molecule itself doesn’t look like much, but its function can make or break entire processes in adhesives, sealants, and coatings. Anyone who has tried to get two stubborn surfaces to stay together under heat, moisture, or chemical exposure recognizes the value of these connectors.
This compound appears as a clear, colorless to faintly yellow liquid. You notice a pungent odor when uncapped, hinting at some volatility and a need for careful handling. Its density lands around 1.06 g/cm³ at room temperature, and it boils just above 190˚C. Chemists respect its reactivity with water, where it doesn’t just dissolve but instead starts breaking the Si–O–CH₃ bonds, releasing methanol and revealing the reactive silanol groups. In the hands of someone unprepared, this reaction can lead to sticky gels or cloudy films, so moisture control in storage and use matters. Flammability sits in the moderate range for organic liquids, and care should be taken to avoid static sparks or open flames anywhere near stored stocks.
Every supplier throws a slightly different purity spec depending on the market, but anything above 98% counts as suitable for most industrial uses. Labels carry warnings about flammable liquid and vapor, plus those diamond-shaped hazard codes everyone grows to recognize in a lab or warehouse. Containers usually hold standardized batch numbers and traceability stickers, and responsible vendors provide certificates of analysis covering residues, moisture content, and exact percentages of main and side products. This level of labeling not only meets regulatory demands but allows anyone using the product to double-check sources and cross-reference for quality or troubleshooting.
Industrial production involves reacting phenyltrichlorosilane or phenylsilane with excess methanol under controlled conditions. Temperatures get monitored closely because side reactions generate hydrochloric acid or hydrogen gas. Skilled workers rely on batch reactors fitted with scrubbers and cooling jackets—safety equipment designed from decades of lessons learned the hard way. Yields often surpass 90%, but tweaks to timing, solvent volumes, or reaction temperatures have dramatic effects on the appearance and purity of the final product. Downstream, vacuum distillation and drying steps ensure removal of unreacted alcohols or by-product chlorides, which, if left unchecked, will ruin later applications.
In the lab, phenyltrimethoxysilane reacts with water, alcohols, or amines, giving off methanol and forming Si–O–Si linkages. Makers of advanced materials value its role as a coupling agent, letting hybrid networks form between organic coatings and mineral substrates like silica, alumina, or even certain metals. Once bonded, these networks help coatings cling tight, improving resistance against peeling or chemical attack. Some research teams explore modified forms bearing various functional groups on the phenyl ring, looking for ways to fine-tune grip, add sensing abilities, or boost UV resistance without sacrificing the basic anchor function that forms the heart of the chemistry.
In commerce and research catalogs, names change with jurisdictions and suppliers. Some call it phenyltrimethoxysilane, others use trimethoxy(phenyl)silane. In older literature or according to European conventions, the term PTMS crops up frequently. Each name refers to the same core molecule, though idiosyncratic labeling by specific brands or distributors means buyers do best by cross-checking CAS numbers and structural formulas before ordering in bulk.
No one in the industry takes safety around alkoxysilanes lightly. Inhalation of vapors or skin contact can irritate, and the risk rises if methanol gets released during hydrolysis, especially in poorly ventilated workspaces. Facilities use local exhaust, full-length gloves, and goggles for anyone pouring or transferring the stuff. Standard operating procedures stress spill readiness, vapor monitoring, and regular equipment checks, preventing small leaks from becoming costly accidents. Regulations in Europe, the United States, and Asia keep shifting as new data emerges, so compliance officers invest time and money to stay current, updating training and documentation accordingly.
Most of us never think about what makes modern coatings last, but anyone maintaining an industrial site does. In silane-treated paints, PTMS forms chemical bridges between glass fibers and polymer matrices in composites, giving wind turbine blades their durability and high-end golf clubs their snap. Electronics fabricators use it for surface modification, where it enables the creation of micro-patterns for chips and sensors. Sealant makers rely on its hydrophobic traits when formulating water repellents for concrete, stone, or antique masonry. It even pops up in the pharmaceutical sector as a surface modifier for labware, letting biological samples spread thin and even on diagnostic slides. Each use case grew from hard-earned industrial experience, as labs and manufacturers chased better performance without ballooning costs.
A lot of what we know about PTMS chemistry comes from decades of trial and error, as well as focused measurement. Researchers keep finding new wrinkles to exploit—for instance, grafting PTMS onto particles to improve dispersion in polymers or creating advanced membranes that separate gases more efficiently. Nanotechnology teams play with silanation recipes that yield biosensors sensitive enough to spot a single virus. Environmental engineers look at how PTMS-based treatments might protect infrastructure against acid rain or salt spray. Each incremental gain stems from people in crowded labs testing every variable, jotting rough notes, and sharing those insights through conference talks and open-source preprints.
While PTMS doesn’t rank at the top of toxicity charts, the methanol it liberates during use absolutely does. Methanol poisoning remains a real risk in unventilated or poorly managed settings. Toxicologists track exposure limits carefully, noting that direct skin contact can cause dryness or dermatitis, and that inhaling vapors could mean headaches or, at higher doses, nervous system effects. Chronic studies in animals show little strong evidence of cancer risk at normal exposure levels, but regulatory agencies keep the pressure on to limit mistakes and monitor worker health. Every safety data sheet now comes packed with emergency instructions, reinforcing that while the chemistry opens doors in material science, it comes with clear responsibilities for those using and storing the product.
Thinking years ahead, the direction for PTMS points firmly toward greener, safer, and higher performing materials. Research groups investigate routes that avoid hazardous solvents, trim waste, and extend product lifespans. The push for sustainability means more work for recycling composite materials or breaking down silane-based coatings at end of life. In electronics, as devices shrink and demand sharper patterning, chemists look for ways to modify PTMS molecules to layer even thinner films or build circuits that consume less power. Every large manufacturer feels the pressure to deliver more for less, while academic collaborators look to tailor molecule design for new markets in energy, healthcare, and climate protection. The journey continues, driven by those who balance curiosity with caution, solving problems with creativity and patience.
Out in the world of chemistry and manufacturing, phenyltrimethoxysilane doesn’t make headlines, but it sure handles important work. You’ll find it mostly where materials cross paths with technology — glues, protective coatings, even electronics. The full name might seem a mouthful, but the story behind it is pretty straightforward.
Imagine you’re dealing with a material that needs to stick to something totally different — like a glass-fiber piece joining up with a synthetic rubber. That connection doesn’t just happen on its own. Phenyltrimethoxysilane plays the role of a middleman. The molecule grabs onto the glass surface with the silicon part, and latches onto organic components with its phenyl group. This means fewer headaches later with peeling layers or weak connections. Vehicle manufacturers and makers of electronics want these bonds to hold up under stress, sunlight, and heat. A good surface treatment makes all the difference between a gadget that survives moisture and one that fizzles out before the warranty ends.
Anyone who’s seen what water can do to concrete, glass, or stone knows the toll of weather isn’t always slow. The waterproofing market banks on additives that can penetrate deep into building materials, keeping moisture out. Silanes like phenyltrimethoxysilane get absorbed into the pores of stone or concrete and form a shield at the molecular level. Water beads up and rolls off, instead of seeping in. Homeowners and builders avoid a ton of repair costs this way, and cities keep their bridges looking sharp longer. Studies back this up — treated surfaces soak up much less water, and that’s not just cosmetic. Less water means lower freeze-thaw damage and less corrosion of steel reinforcements.
Modern electronics lean on materials that don’t just conduct electricity, but also block unwanted static, moisture, or heat. That’s where silanes walk in, not just as a chemical add-on, but as a way to keep complicated devices running trouble-free. In semiconductors and circuit boards, phenyltrimethoxysilane helps get surfaces ready for more chemical treatments. It can tweak how chips are etched and how films stick to each other, adding reliability. This sort of chemical fine-tuning becomes more important as devices shrink and parts cram together more tightly.
Silicone rubber and resins also get a lift. Adding phenyltrimethoxysilane during production improves transparency, heat resistance, and mechanical strength. Anyone who’s worked on sealants for aquariums, insulating windows, or heavy-duty kitchen tools knows how crucial durability can be. Most folks just want something that works, and this molecule helps manufacturers deliver exactly that.
Chemists have raised concerns about volatile organic compounds (VOCs) coming off traditional silane treatments. Safer application processes and tighter workplace controls have answered some of these worries. The push continues for greener manufacturing, not just to tick regulatory boxes, but because workers, neighborhoods, and buyers all expect cleaner, safer materials. The challenge sits in balancing performance with environmental health. On this front, teams behind the scenes push toward less-tinted, lower-emission formulas that get the job done.
Folks searching for better bonds or longer-lasting surfaces don’t usually ask questions about chemical structures, but they sure notice when things last longer, look better, or simply just work. Phenyltrimethoxysilane plays a reliable part in many of those small victories, right from the lab bench to the sidewalk.
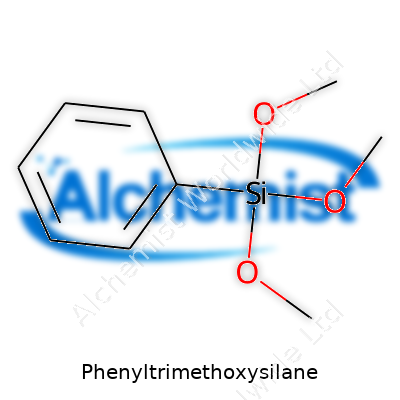
Walking through any chemistry lab or material research facility, it’s easy to spot a few bottles labeled with technical-sounding names. Phenyltrimethoxysilane often shows up in those rows, and for good reason. Its chemical formula, C9H14O3Si, packs a punch both in research and in industry. Breaking it down, this compound has a silicon atom at its core, attached to a phenyl ring and three methoxy groups. That structure gives it unique properties, making it valuable for all sorts of applications, including as a surface treatment or in the making of advanced materials.
Some folks won’t think too much about a formula like C9H14O3Si. But that small cluster of elements shows up in projects that reach into electronics, coatings, even medical devices. The phenyl group tacked onto the silane brings a bit of aromatic stability, and those methoxy groups create possibilities for reacting with other materials, especially stuff like glass or metal oxides. I’ve seen it used on old lab benches to treat surfaces, changing a simple glass slide into something water and oil repel. That trait doesn’t happen by accident—it comes from this exact chemistry.
Safety doesn’t take a backseat here. Knowing the composition isn’t about memorizing for a test, but about understanding how this silane can hydrolyze and release methanol, a toxic alcohol, when exposed to water. Wearing gloves, having good ventilation, and storing these compounds tightly sealed makes all the difference. Recognizing the formula lets chemists plan for risks, not just rewards.
From tech startups to deep-pocketed manufacturers, C9H14O3Si gets sprinkled into a lot of unexpected projects. In coatings on microchips, this silane helps keep circuits free from stray moisture and dust, which in turn keeps modern electronics ticking. I’ve watched engineers debate the best silane for their composite materials—phenyltrimethoxysilane’s unique bond with glass fibers can tip the balance for a longer-lasting product.
In medical device development, making sure surfaces don’t promote unwanted bacterial growth can be a game-changer. This silane doesn’t just stick stuff together. It can form chemical bridges that change how the surface interacts with its environment. Leaning on that formula, scientists keep finding ways to control which molecules stick and which ones slide away.
Questions come up every time new material hits the market: Is it safe enough? Can it be made with fewer hazards? With phenyltrimethoxysilane, teams focus on both the big benefits and the finer points of safety. More research goes into making sure byproducts like methanol get contained and recycled. Training workers doesn't stop at handling: it covers clean-up, storage, and emergency response. Sharing data about long-term exposure or environmental impact could help the next generation make smarter choices, too.
Small molecules like C9H14O3Si hide complex stories. Chemistry drives progress in technology and industry, but it takes informed users to balance performance with responsibility. Knowing the formula is the first step, not the finish line.
Phenyltrimethoxysilane isn’t your average chemical. People use it in everything from silicone sealants to coatings on electronics. Because the chemical comes with hazards, a little care and planning can have a big impact on health and safety.
When dealing with Phenyltrimethoxysilane, it helps to know what you’re up against. Direct contact can burn skin and eyes. Vapors bother the respiratory tract and sometimes leave headaches. Its flammability can turn mistakes into disasters. Once, I watched someone set a beaker down near a hot plate—they didn’t know about the vapors, and the room filled with fumes in seconds. That stuck with me. Chemicals don’t always announce themselves, but the consequences are hard to ignore.
A storage area changes everything. I’ve noticed people sometimes treat chemical containers like boxes of cereal, stacked in a storeroom. That’s not just careless; it’s risky. This chemical breaks down when water sneaks in, and the byproduct—methanol—brings its own hazards.
Store every container in a spot that stays dry and cool. Humidity opens up the risk of leaks and reactions. Ventilation matters too—the air should move, not stagnate. Metal cabinets with fire ratings, designed for flammables, cut down fire risk. Labels should always face out so nobody guesses what’s inside. Once someone replaced a corroded lid with a soda bottle cap. Bad idea. Use original packaging with tightly sealed lids.
Try skipping gloves or goggles even once, and you’ll feel the regret. Chemical splash burns linger. Gloves made of nitrile or butyl rubber block the liquid. Goggles or face shields protect eyes. A simple lab coat saves skin, and if there’s a spill, you can shed it fast. For folks who spend hours handling chemicals, adding a respirator with organic vapor cartridges removes a hidden risk. Time after time, protective gear makes the difference between a close call and a trip to the doctor.
Keep incompatible chemicals apart—acids and phenyltrimethoxysilane won’t play nice together. Always measure and pour in a fume hood whenever possible. Keep fire extinguishers handy and easy to reach; check that they’re rated for chemical fires. Writing up a checklist helps, especially if handling goes on in shared spaces.
Accidents show up suddenly. If a spill happens, get everyone out and vent the space. Use absorbent pads that stand up to solvents, not paper towels. Dispose of the mess as hazardous waste—never dump it down the sink. Get medical help if anyone gets exposed. An up-to-date safety data sheet should always be on hand. In companies where I’ve worked, reviewing emergency steps before starting always kept the team safer.
Proper ventilation, solid labeling, personal protection, and regular training build habits that last. Newcomers often learn the hard way, but I’ve seen teams reward those who call out shortcuts. The goal is simple: go home at the end of the day with the same health you came in with. Following proven guidelines from OSHA and chemical manufacturers never feels like a burden once you’ve witnessed what goes wrong when they’re ignored.
Anyone who has spent time in a lab or a facility that works with silanes knows there’s a lot more to worry about than a chemical’s technical data sheet. Phenyltrimethoxysilane, often used to tweak the surface properties of materials or to help create silicone polymers, can turn into a genuine safety headache if people aren’t mindful. Once that bottle comes out of storage, the gamble starts. The clear, colorless liquid might look harmless, but don’t let that fool you.
If you’ve ever opened a container and caught the harsh, biting odor from compounds like this, you know how quickly fumes can sting the nose and throat. Even a short breath in an unventilated space can irritate respiratory passages. Lab notes and safety data sheets point out that repeated exposure isn’t just uncomfortable — it brings coughing, headaches, and sometimes more severe breathing issues. It’s not just about the air. Getting Phenyltrimethoxysilane on skin or in the eyes burns and blisters fast. I remember once seeing a small splash cause a colleague’s skin to redden within minutes — and nobody had much doubt afterward about why gloves and goggles matter.
It doesn’t play nice with water. Just a few minutes in a humid environment and you start noticing the sharp odor of methanol forming. Methanol release is no minor issue — it’s toxic by inhalation and absorption, and in a spill, the invisible vapors creep along the ground. This puts people at risk of symptoms such as dizziness, nausea, and, in larger exposures, much worse. Methanol is also highly flammable. That means that something as innocent-looking as a damp rag or an unnoticed puddle on the bench could ignite if a spark comes along.
The health conversation doesn’t end once the smell clears out. Chronic exposure raises concerns about effects on the liver or nervous system, since both silanes and methanol can build up in the body with time. Cases of prolonged handling without the right precautions don’t usually turn out well. Some researchers have spoken about headaches that will not fade, chronic cough, or even numb fingers. The science isn’t always settled, but those personal stories build a pattern: taking these risks seriously pays off in the long run.
Far too many workplaces hope that a fume hood and a pair of goggles will do the trick. In reality, people need to pay close attention to how Phenyltrimethoxysilane is stored, transferred, and disposed. Keeping storage containers tightly sealed and away from dampness matters. Ventilation isn’t optional, and relying on a single exhaust fan misses the bigger picture. Getting spills under control quickly, before they spread or react with something else on the bench, is a habit everyone should learn early.
It helps to run regular safety drills. Knowing where the nearest eyewash or emergency shower stands can make the difference. And in labs I’ve worked in, tracking chemical inventories closely means outdated stocks don’t sit forgotten — less risk for accidental exposure or breakdown. Even simple checklists at shift changes cut down on overlooked bottles or half-closed caps.
People sometimes ask if safer alternatives exist. For many uses, it’s possible to switch out hazardous silanes for products that don’t throw off toxic byproducts when handled improperly. This takes real cooperation between purchasing, lab supervisors, and even external suppliers. Where substitutions can’t work, better protective gear — and actual buy-in from everyone onsite — reduces daily exposure risks.
Dealing with chemicals like Phenyltrimethoxysilane leaves no space for shortcuts. Knowledge, vigilance, and the right safeguards keep people healthy. It only takes one mistake to learn why these measures matter so much — and too many people find out the hard way.
Working in materials science, I’ve learned that coupling agents like phenyltrimethoxysilane often make or break the bond between organic polymers and inorganic fillers. Plenty of formulators reach for silanes because of their ability to improve the adhesion and durability of composite materials. The question many face is: can phenyltrimethoxysilane be safely blended with other chemicals in multi-silane systems without risking unpredictable reactions, poor shelf life, or plain wasted material?
Chemically speaking, phenyltrimethoxysilane offers a phenyl group connected to silicon with three methoxy groups ready for hydrolysis and condensation. This setup means the material attaches well to glass and silica surfaces while offering good compatibility with organic matrices like resins. What stands out in practice is that the phenyl group provides a bump in hydrophobicity and thermal stability compared to alkyl silanes like methyltrimethoxysilane.
The trouble rarely comes from phenyltrimethoxysilane itself, but from the other ingredients in the pot. Many resin systems, like epoxy or urethane, often use amino or epoxy-functional silanes. Mixing those with phenyltrimethoxysilane usually helps tune surface energy and boost aging stability. I’ve seen labs deliberately combine them to strike a balance between mechanical strength and moisture resistance. Field tests have shown that blends containing both amino and phenyl silanes outlast single-silane formulations, especially outdoors.
Hydrolysis behavior throws up a warning flag. Silane coupling depends on hydrolyzing the methoxy groups, which generate methanol—and these reactions differ depending on pH, temperature, and water content. Problems often pop up when people mix silanes with different groups that hydrolyze at much different speeds. If you prepare a blend and one silane condenses too fast, it can leave the rest behind, leading to uneven surface treatment, flaking, or sticky residue.
Formulators usually avoid the biggest headaches by using mild acid catalysts, keeping solutions dilute, and mixing right before use. If you’re adding phenyltrimethoxysilane to a blend, keep an eye on compatibility with strongly basic or nucleophilic ingredients, since these sometimes cause unwanted crosslinking. Basic amino silanes in the same pot, for example, can sometimes react with condensed phenyl silane groups before they even reach the substrate, especially if water is around.
I’ve found that staggering the mixing order solves a lot of headaches. Hydrolyze and blend the silanes under the same pH and solvent conditions, then quickly apply to the substrate. Don’t store mixed solutions for too long, unless you want to come back to a gummy mess. Always run compatibility checks if working with new resins or unusual co-monomers, even if literature promises success—laboratory results still trump theory.
Working with suppliers who document the purity and side-product profiles of their silanes makes troubleshooting easier, too. Adding a little extra drying agent—like freshly activated molecular sieves—keeps water out of storage barrels, extending shelf life and performance. For anyone scaling up, pilot runs matter. Even minor contaminants or shifts in ambient temperature can throw off the final product in surprising ways, so it pays to catch problems early.
Experience shows that phenyltrimethoxysilane fits into many multi-silane recipes as long as water content, pH, and order of addition stay under control. Field experience and hands-on tests still count for more than theoretical guidelines. Keeping open communication with suppliers and pilot-line operators helps iron out snags before full production, keeping both performance and safety where they belong.
| Names | |
| Preferred IUPAC name | (trimethoxysilyl)benzene |
| Other names |
Trimethoxyphenylsilane Phenyltrimethoxy silane PTMS Phenyltrimethoxysilane (PTMS) |
| Pronunciation | /ˌfiː.nɪlˌtraɪˌmɛθ.ɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 2996-92-1 |
| Beilstein Reference | 1465063 |
| ChEBI | CHEBI:51799 |
| ChEMBL | CHEMBL1389986 |
| ChemSpider | 12271 |
| DrugBank | DB14096 |
| ECHA InfoCard | 100.034.135 |
| EC Number | 213-677-5 |
| Gmelin Reference | 82377 |
| KEGG | C19747 |
| MeSH | D010622 |
| PubChem CID | 66205 |
| RTECS number | VV9275000 |
| UNII | W9Z05XQS0V |
| UN number | UN1992 |
| Properties | |
| Chemical formula | C9H14O3Si |
| Molar mass | 198.29 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Aromatic |
| Density | 0.97 g/mL at 25 °C (lit.) |
| Solubility in water | Insoluble |
| log P | 2.8 |
| Vapor pressure | 0.5 mmHg (25 °C) |
| Acidity (pKa) | 7.4 |
| Basicity (pKb) | -3.5 |
| Magnetic susceptibility (χ) | -59.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.450 |
| Viscosity | 1 mPa·s (25°C) |
| Dipole moment | 2.20 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 352.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -368.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1763.7 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H302, H319, H332, H335 |
| Precautionary statements | Precautionary statements of Phenyltrimethoxysilane: "P261, P264, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364, P403+P233, P405, P501 |
| Flash point | 76 °C |
| Autoignition temperature | 410 °C |
| Explosive limits | Explosive limits: 1.1–9.7% |
| Lethal dose or concentration | LD50 (oral, rat): 2200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, Rat: 1757 mg/kg |
| NIOSH | GZ1550000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Phenyltrimethoxysilane: "Not established |
| REL (Recommended) | 100 ppm |
| Related compounds | |
| Related compounds |
Phenylsilane Phenyltriethoxysilane Methyltrimethoxysilane Vinyltrimethoxysilane |