
Propyl silicate grew out of the early 20th-century surge in organosilicon research. Chemists started to notice the immense chemical potential of silicate esters. With the natural abundance of silica and the rising global demand for specialty chemicals, the path toward alkoxy silicates like propyl silicate became well-trodden by industrial pioneers. Processes improved slowly as chemical labs, first in Europe and then across North America and Asia, unlocked the methods to harness silicate esters in coatings, sealants, and surface treatments. In my work with specialty coatings, the transformation in performance and application speed that came with the commercial use of propyl silicate stood out. Factories no longer handled only natural silicates or early, less stable esters; by the late 1970s, they relied on the far more predictable outputs propyl silicate provided. The shift brought cleaner production lines, longer shelf lives, and, crucially, new material science advances.
Propyl silicate, often sold as tetra-n-propyl orthosilicate or just TPOS, arrives as a clear, flammable liquid with low viscosity. In industrial shipments, it usually comes in airtight drums, labeled to highlight its flammability. My work in a coatings lab revealed its knack for blending with other organosilicates. Manufacturers favor TPOS because it helps build strong, silica-rich networks during hydrolysis and condensation steps in sol-gel processes. At the point of use, TPOS delivers silica in a reactive, highly dispersible form—this opens up a suite of uses from moisture scavenging in sealants to acting as a crosslinker in films.
Propyl silicate weighs in at around 1.008 g/cm³, and its boiling point nudges just above 213°C. It's colorless and nearly odorless, offering a faint, sweetish scent. Solubility presents an interesting challenge—it breaks down under the influence of water but comfortably dissolves in ethanol, toluene, and most organic solvents. The compound hydrolyzes quickly, especially with acids or bases present. Anyone working in coatings or sealants learns fast how TPOS can ruin a batch if humidity spikes.
Regulations push suppliers to offer propyl silicate in high purity, above 98%. The packaging sports the UN 1993 code for flammable liquids, and features a health hazard warning tied to potential toxicity. Technical sheets detail the silicate content, hydrolyzable silica percentage, and water content. Every canister I received included a batch quality certificate—customers never trust a mystery mix for high-stakes industrial applications.
Most propyl silicate hits the market after direct reaction of silicon tetrachloride and n-propanol, with a base or acid catalyst to push the exchange. Experience in glass labs showed me how moisture becomes a menace: water triggers premature hydrolysis, wrecking yields by forming gels before the ester escapes purification. Manufacturers keep the process closed and inert, and distill the product under reduced pressure. Governments, for their part, limit impurities, especially silanols and residual halides, which can produce corrosive by-products downstream.
Propyl silicate loves to hydrolyze, trading its propoxy groups for Si-OH bonds and releasing alcohol. In manufacturing lines, operators control temperature and add precise water doses to steer the sol-gel reaction. It reacts with bases and acids, throwing off varying degrees of silica and forming intricate Si-O-Si networks used in glassy coatings. TPOS serves as a handle for attaching other functional groups—researchers tweak its alkoxy groups to adjust reactivity, gel time, and mechanical strength of the products. This flexibility keeps it in demand for specialty glass, microporous membranes, and electronics encapsulation.
Commercial supply circles refer to propyl silicate as tetra-n-propyl orthosilicate, n-propyl silicate, TPOS, or silicic acid, tetrapropyl ester. Large chemical suppliers carve out their own brand names—one might see products like Sipernat, Dynasil, or Ormosil silica precursors on bulk orders. Confusing jargon can bite the unwary engineer; suppliers use naming conventions rooted in old patents, but chemical structure stays constant.
Workers fight two key hazards: flammability and acute toxicity. Propyl silicate’s low flash point—about 56°C—makes fire risk ever-present in manufacturing plants. Fume hoods, explosion-proof storage, and strict maintenance cut down on hazard. On top of fire, inhalation burns and nervous system effects have sent lab techs to the hospital, pushing companies to require NIOSH-approved respirators and spill kits. Firms enforce closed processes and automatic dispensers, so at no point does a worker face a splash or large spill without protection. My safety officers drilled us to treat leaks as emergencies, not minor inconveniences.
Most propyl silicate ends up in sol-gel chemistry, where it acts as the silicon donor for glass films, ceramics, and reinforcing fillers. One critical niche: advanced protective coatings on steel or aluminum, where only strong covalent Si-O bonds block corrosion and wear. In electronics, TPOS enables high-purity silicon layers for semiconductor passivation. Some specialty cements, built for oil wells or chemical plants, also tap into TPOS’s ability to deliver hard, water-resistant silica in situ. The compound anchors adhesion promoters onto industrial surfaces and helps build transparent, tough sealants. Over two decades, I’ve seen TPOS step into new roles, such as moisture scavenging in hermetic packaging, or forming the backbone of protective glasses for harsh marine environments.
Innovation with propyl silicate often focuses on hybrid materials. Scientists mix it with metal alkoxides to craft new sol-gel composites—these experiments unlock polymer-reinforced glass films or flexible ceramic coatings. Startups working in nanotechnology find value in TPOS as a precursor for highly uniform silica nanoparticles. The push to lower processing temperature appeals to both electronics firms and energy plants looking for new surface treatments that cut energy costs. I’ve worked with teams investigating new crosslinked gels for battery separators; TPOS easily fits into these advanced composite structures, bestowing thermal stability and mechanical stiffness.
Toxicologists have spent time dissecting the dangers of propyl silicate. Acute exposure triggers lung and eye irritation, and workers have reported headaches and dizziness from solvent vapors. Animal studies report chronic exposure risk to kidneys and liver at high doses. Propyl silicate breaks down by releasing propanol and silicic acid; while the final breakdown products look innocuous in environmental terms, the immediate toxicity keeps industry on high alert. I always trusted tight environmental controls in refineries and labs, knowing the cost of a major release far outweighs any production savings. Occupational exposure limits stay conservative. Emergencies require fast first aid, including eye washes and special burn treatment for chemical splashes.
Looking ahead, TPOS seems poised to stay essential in next-generation hybrid materials. Researchers chase silicon precursors with cleaner handling and even higher purity, searching for silicates that cut the need for aggressive solvents. Environmental pressure nudges suppliers to design recycling schemes or push for greener production paths. Nanomaterial labs prize TPOS for precise control in nanoparticle assembly—a trend that keeps growing as flexible electronics and lightweight composites take center stage. My colleagues and I keep a close eye on new toxicity data and look for process tweaks that squeeze efficiency from older chemistries. If history repeats, the next evolution may not wipe out TPOS, but push it into still more demanding, specialized roles that shape the future of electronics, energy storage, and smart coatings.
Most people won’t find a bottle labeled “propyl silicate” at the hardware store or local pharmacy, yet this chemical quietly supports everything from bridges to fine art. After a recent weekend patching up garden furniture, I found myself looking deeper into the tough coatings I’d used — and propyl silicate kept popping up in the fine print. That got me thinking about where else it lives beyond a can of paint primer.
Propyl silicate is a liquid used mostly as a binding agent in products that need to stand up to harsh conditions. Manufacturers rely on it in paints, coatings, adhesives, and sealants, especially for metal surfaces. It locks down molecules to form a tough layer that resists heat, water, and chemicals, which keeps steel from rusting away before its time. For industrial infrastructure, there’s a real cost to corrosion, with the American Galvanizers Association estimating that the U.S. spends billions yearly fighting the problem.
I remember a cousin working as a welder at a shipyard, talking about how metal beams would rust out faster if not for the right primer. The shipyard coated sections with silica-based binders like propyl silicate, so ships could tackle waves and salt without falling apart by the next dock inspection. In paint manufacturing, it acts as a bridge between pigments and surfaces, holding color where it belongs on ships, bridges, pipework, and even in automotive primers.
Conservators and artists have picked up propyl silicate as a tool for fixing ancient frescoes, marble sculptures, and mural walls. The material seeps into worn stone or flaking paint and locks everything in place. A friend who works at a city museum described how tricky restoration can get. They need materials that won’t yellow, chip away, or cause more damage years later. Propyl silicate’s transparency and stability make it a safer choice compared to some older, harsher chemicals. Studies from the Getty Conservation Institute point out its positive record in protecting old architecture, especially important in places prone to pollution and acid rain.
All chemicals have trade-offs. Propyl silicate works wonders where durability matters, but it doesn’t come without caution. Straight out of the drum, it can irritate eyes, skin, and lungs. Industrial workers follow safety guidelines, from wearing gloves and masks to making sure areas stay well-ventilated. If mishandled, the chemical can leak into water or soil, so plant operators and regulators keep a close eye on storage and disposal. The good news is propyl silicate breaks down over time and hasn’t shown the same long-term toxicity as heavy metals or persistent organic pollutants. Scientists working with the EPA continue to track its impact to keep factories from trading one environmental headache for another.
Everyone benefits when companies share more about the ingredients hiding in paints and coatings. High standards bring safer practices. As someone who loves home improvement, I keep an eye on eco-labeled products and updated safety sheets before picking up my brushes. For larger industries, there’s ongoing research into water-based binders and less hazardous alternatives. Researchers at universities, along with companies, push for solutions so that strong protective coatings don’t have to mean health or environmental risks — the balance isn’t perfect yet, but progress comes from keeping these questions up front.
Propyl silicate finds its way into a range of industrial settings. Paints, coatings, adhesives, and certain construction materials rely on it for binding power and strength. Many folks who work in manufacturing, especially those pouring paints or blending finishes, meet it daily. The substance usually goes by its chemical label: tetrapropyl orthosilicate or TPOS. It seems pretty technical, but for a lot of workers, it’s just part of the job.
Questions about safety crop up for good reason. Workers and those living near factories deserve honest information about what’s in the air. Propyl silicate isn’t as familiar as lead or asbestos, but that doesn’t mean it should slide under the radar. Exposure usually comes in two ways: you breathe it in, or it touches your skin. The liquid form gives off vapors. Without the right gear or proper ventilation, these vapors travel into your lungs.
Research points to eye irritation and nose or throat discomfort when people handle propyl silicate without protection. Skin contact can lead to redness, itching, or in unlucky cases, chemical burns. Nobody wants to risk their eyesight or breathing. The stories from those who’ve worked with industrial solvents tell enough. You mix paint or clean tools, and pretty soon your throat feels dry, your eyes water, or your skin breaks out in rashes. These symptoms don’t fade if the exposure continues.
Looking beyond the immediate effects, chronic exposure draws more cause for worry. Some silicates linked to lung fibrosis and other respiratory troubles. The lungs can’t handle foreign chemicals year in, year out. The World Health Organization and OSHA flag propyl silicate as a potential hazard. The short-term effects stack up over time, especially if proper workplace habits slip. At this point, there’s limited evidence about propyl silicate causing cancer, but the lack of clear data shouldn’t mean ignoring early warning signs.
I’ve seen workers get casual about “lesser-known” compounds — no mask, no gloves — thinking it’s fine compared to harder stuff. As soon as dizziness or skin problems show up, everyone wonders if it’s worth the risk. Any chemical that can mess with your lungs or skin in the short run has the potential to do more harm over months or years.
Factories and small workshops both need to respect the risks. Clear labeling on drums, good training, and enforced use of gloves and respirators go a long way. Doesn’t help to hide warnings on the second page of a manual. Ventilation, real-time monitoring for vapor levels, and running air filters can drop risk sharply. People should know symptoms to watch for, so problems get caught early. Managers should not take short-cuts, and workers should feel able to step back and demand safety controls.
For consumers, worries about dried paint or coated surfaces tend to be low. The bigger hazard lands on the people who mix, pour, or spray the product. That means the law and workplace habits have a bigger say in protecting community health than individual choices. Company leaders owe it to their teams to follow the science, not just the letter of regulations.
Real safety comes from honest communication, up-to-date protective gear, and respecting health warnings — even for chemicals you don’t hear about on the news. Governments update standards with new data, but those on the shop floor know what symptoms to trust. Nobody works well or lives well with headaches, coughs, or burned skin. Those risks deserve attention. PPE, training, and good ventilation are not extras — they’re the baseline for anyone handling propyl silicate.
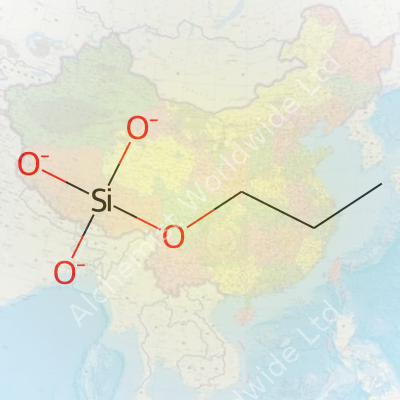
Silicon compounds show up everywhere, from smartphone screens to car parts. Propyl silicate joins that list, though most folks don’t see it at the grocery store. In the world of chemistry and materials, this compound plays a noticeable role. To answer the big question: the chemical formula of propyl silicate is C9H22O4Si. Chemists recognize it as tetrapropyl orthosilicate or simply tetrapropyl silicate. The structure comes from attaching four propyl groups to a central silicon atom, each connected through oxygen atoms.
Propyl silicate doesn’t live on a lab shelf collecting dust. You've got paint on your wall? Tough coatings on your tiles? Chances are, compounds like propyl silicate help hold them together. Its ability to form strong, durable films turns it into an ingredient in adhesives, surface finishes, and sealants. In my experience working with materials, the use of strong yet flexible bonding agents separates a product that lasts from one that cracks and chips away. Synthetic chemistry has made everyday items resilient, and propyl silicate fits that mission.
Digging into the formula, silicon bonds to four oxygen atoms, and each oxygen links to a three-carbon (propyl) group. This layout creates a molecule with both organic and inorganic properties. That dual nature matters. The propyl groups help the compound dissolve in organic solvents, which makes it easier to mix into varnishes and surface treatments. At the same time, the silicon-oxygen bonds become the backbone in forming a strong silicon dioxide (SiO2) network when the material cures.
From what I've watched in lab demonstrations, this transformation opens the door to coatings that repel water, resist wear, and grip tight to metals or glass. For industries that deal with extreme heat or harsh environments, coatings based on propyl silicate hold up when many glues or paints would break down.
Products that create robust industrial coatings often bring safety and environmental questions. Propyl silicate can irritate the skin, eyes, and lungs if handled carelessly. The hydrolysis reaction, which is where the compound meets water and eventually forms silicon dioxide, releases alcohol vapors that can be harmful indoors without proper ventilation. In an old lab where I interned, a few minutes with these vapors made a standard mask feel useless.
Solutions do exist. Gloves, eye protection, and fume hoods offer real protection for workers, reducing health risks by limiting exposure. Switching to less hazardous silicates or inventing new binding agents could ease the burden in the long term. Regulations now require clear labeling and strict handling protocols, which helps prevent careless mistakes in the workplace.
Anyone working in manufacturing, research, or even restoration of historic buildings gains an advantage from understanding a simple formula like C9H22O4Si. Knowing what’s in a compound, and how it reacts with everyday materials, arms builders and designers with information for making safer and better products. For me, this practical knowledge often separates a rushed shortcut from work that stands the test of time.
Propyl silicate plays a hidden but important role across plenty of manufacturing and chemical industries. People might not talk about it at dinner tables, but for the folks who work in labs, paint plants, or electronics processing, mishandling it can wreck expensive equipment or, worse, put health at risk. I’ve seen more than one workplace scramble to contain a leak or clean up unexpected crystallization, all because the right steps weren’t taken from day one.
This liquid likes to stay out of the limelight. Exposure to moisture can turn it cloudy and eventually degrade its quality. Left open to the air for too long, it will break down, releasing alcohol and silica dust—a headache for anyone downwind. That’s not just a waste, it can be dangerous.
From my own experience, it pays to avoid plain old glass bottles. Despite sounding sturdy, they rarely come with trusted seals, and fine cracks or chips can invite disaster. Stainless steel or high-density polyethylene containers with secure, tight-fitting lids fare much better. Industry experts, including OSHA and the European Chemicals Agency, recommend this route. Not just because it preserves the product, but because it keeps fumy leaks to a minimum.
Ask any lab manager and they’ll tell you—temperature control isn’t a luxury, it’s a necessity. Leaving propyl silicate near heat sources or windows causes it to break down faster, sometimes releasing ethanol vapor. Keep it in a cool, well-ventilated spot, out of direct sun. I always opt for flammable materials cabinets designed to vent vapors safely, a tip passed to me by an old mentor after we nearly lost a storeroom months shy of an inspection.
Humidity ranks high on the enemy list. Moisture sneaks in through careless caps or through the air itself, which can kickstart unwanted reactions. Desiccants or sealed secondary containment reduce the chances of water finding its way in. In one facility I visited, simply moving the product from an open shelf to a sealed drum cut spoilage rates almost to zero.
Bulk containers must be clearly labeled. It’s a mistake I’ve watched someone make—markings faded after months, contents got confused with another solvent, and drama followed. Taking five minutes to double-check chemical labels and update them each time a new shipment arrives can save days of work and loads of money.
Fire risk can’t be ignored. Propyl silicate isn’t just flammable, its vapors are heavier than air and can drift to an ignition source. Grounding storage drums and shelving keeps static electricity at bay. Proper spill kits should always sit a few steps away—absorbent pads, gloves, goggles, and a way to contain leaks.
Written safety guidelines are only as effective as the team following them. The healthiest work cultures I’ve seen treat storage and handling as a living routine, not just a monthly box to tick. Regular refreshers and reminders help, especially when new workers sign on or management updates their protocols. That kind of shared vigilance forms a safety net around chemicals like propyl silicate, stopping problems before they start.
Investing in purpose-built chemical storage cabinets works out cheaper in the long run. Pair this with clear, practical signage and accessible material safety data sheets. Bringing in a specialist, even once a year, can find gaps most staff would never spot. All these steps cost less than a recall or remediation, not to mention the value of keeping people safe and retain product quality.
Any engineer who’s worked near the sea recognizes the threat that saltwater poses to concrete and steel. Bridges, docks, tunnels—these aren’t just concrete blocks. Waves, rain, freeze-thaw cycles, and air pollution constantly test the limits of these materials. Propyl silicate walks into the scene as a silent guardian. Its chemistry lets it seep into concrete, reacting with moisture to form a glassy network. This hard layer helps slow down water and salts before they corrode rebar. Cities like San Francisco and Hong Kong have treated essential bridges with silicate-based sealers for decades. In the long run, maintenance costs drop and repairs can wait.
Nobody wants to repaint steel railings or machinery every season. Factories and infrastructure managers count on coatings that resist chemicals, heat, and weather. Propyl silicate blends into zinc-rich primers and industrial paints. It reacts as it dries, locking pigments and metals into a tough shell. Factories use these coatings on oil tanks, ship hulls, railings, and power plants. They slow rust and chalking, especially in harsh sun, salty air, or when chemicals threaten to strip bare surfaces.
The casting industry can’t function without mold cores made from sand. Sand shifting or breaking means warped engine blocks and wasted time. Propyl silicate acts as a liquid binder for making sand molds in steel and iron casting. After mixing with other hardening chemicals, it locks the grains together. This method stops sand from crumbling under the force of molten metal, leading to smoother castings and fewer production headaches. Plants in Germany and Japan trust silicate binders to help produce everything from car engine parts to hand tools.
Gadgets aren’t all chips and screens—they have to survive accidents and bad luck. Propyl silicate finds its way into protective coatings for circuit boards. It shields delicate components from humidity, dust, and even short bursts of heat. Beyond that, construction teams mix silicate-based pastes into panels and coatings to slow fire spread. Hospitals, data centers, and skyscrapers use these materials inside their walls and ceilings to buy extra minutes for safe evacuation and emergency response.
No chemical acts alone. Propyl silicate requires expert handling—liquid versions can irritate skin and eyes, and fumes in closed rooms threaten workers’ lungs. Switching fully to water-based or bio-derived sealers runs into issues; many have trouble matching the durability and cost of silicate-based options. Some firms now install improved ventilation and training programs for line workers. Research keeps pushing for safer forms, but the trade-off between ease and performance remains. Responsible companies read up on regulations and make changes where possible.
Propyl silicate won’t headline the news, but it plays a quiet role across concrete, paint, casting, and electronics. Each advance—be it a cleaner factory or a longer-lasting bridge—has roots in smart chemistry. As industries race to last longer and waste less, questions about safety, sustainability, and value will keep moving this work forward.
| Names | |
| Preferred IUPAC name | propyl orthosilicate |
| Other names |
Tetraethyl orthosilicate Tetraethyl silicate Ethyl silicate TEOS Silicic acid, tetraethyl ester |
| Pronunciation | /ˈprəʊpɪl sɪˈlɪkeɪt/ |
| Identifiers | |
| CAS Number | 682-01-9 |
| Beilstein Reference | 1461014 |
| ChEBI | CHEBI:8807 |
| ChEMBL | CHEMBL185881 |
| ChemSpider | 21520 |
| DrugBank | DB16669 |
| ECHA InfoCard | ECHA InfoCard: 100.004.249 |
| EC Number | 220-941-2 |
| Gmelin Reference | 88237 |
| KEGG | C14436 |
| MeSH | D011377 |
| PubChem CID | 10913 |
| RTECS number | TD3325000 |
| UNII | U2191A43GM |
| UN number | UN1292 |
| CompTox Dashboard (EPA) | DTXSID5020704 |
| Properties | |
| Chemical formula | C9H22O4Si |
| Molar mass | 208.33 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.970 g/cm3 |
| Solubility in water | Reacts with water |
| log P | 1.64 |
| Vapor pressure | 0.4 hPa (20 °C) |
| Acidity (pKa) | 12.8 |
| Magnetic susceptibility (χ) | -68.0E-6 cm³/mol |
| Refractive index (nD) | 1.383 |
| Viscosity | 2 mPa.s (20°C) |
| Dipole moment | 2.20 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 358.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -936 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1986.7 kJ/mol |
| Pharmacology | |
| ATC code | J02AX12 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 |
| Flash point | Flash point: 45°C |
| Autoignition temperature | autoignition temperature: 370°C |
| Lethal dose or concentration | LD50 (oral, rat): 6270 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 2107 mg/kg |
| NIOSH | SY8400000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Propyl Silicate: "200 ppm (850 mg/m³) TWA |
| REL (Recommended) | 200 mg/m³ |
| IDLH (Immediate danger) | 250 ppm |
| Related compounds | |
| Related compounds |
Tetraethyl orthosilicate Tetramethyl orthosilicate Tetrabutyl orthosilicate Methyltrimethoxysilane Ethyl silicate |