
Tetraethyl orthosilicate, sometimes called TEOS, entered the industrial scene early in the twentieth century. Chemists searching for new binding agents and silica sources turned to organic-inorganic hybrids like TEOS. Its creation answered the need for a precursor that delivers silicon in a controllable, portable form, which early glassmakers and ceramics specialists could not ignore. Over the decades, academic interest grew, and patents began to pile up as researchers explored its route from simple lab curiosity to vital tool for electronics, coatings, and optics. Even now, TEOS keeps finding new fans in fields obsessed with material purity.
TEOS shows up as a clear, colorless liquid. Its main selling point is its knack for reacting gently with water to give silicon dioxide, making it a key ingredient for thin layers of silica in everything from semiconductors to artwork restoration. Well-sealed containers keep it stable for years, and product grades stretch from basic industrial batches to high-purity versions tuned for silicon wafers and optics. Manufacturers label it with details like minimum purity, density, and water content because a few extra impurities can mess up the final product badly. In my experience, a bottle of TEOS rarely gathers dust in labs that deal with chemical synthesis or advanced coatings.
At room temperature, TEOS has almost the same viscosity as water, but it carries a mild, sweet smell that reminds many of ethanol. The compound boils at about 168°C, but its true trick lies in the hydrolysis reaction. Add a dash of water, sometimes just from humidity, and TEOS starts building bonds with oxygen, producing ethanol and ever-growing silica networks. Chemists appreciate its fire hazard rating—flammable but manageable with good lab habits—and they usually keep it in the chemical fridge to cut back on slow, unwanted reactions. Those using TEOS quickly learn to respect its potential to condense into a glassy mass unless handled with care and a clear plan.
Most suppliers ship TEOS with a guaranteed purity above 98%. A typical label lists the lot number, production date, country of origin, molecular weight—208.33 g/mol—, and storage advice. Some add water content and total acid content because those factors hint at the age and likely performance of the batch. In addition, shipping documents often slap safety icons warning about eye and skin irritation. Many labs demand a certificate of analysis accompanying each drum, especially when results depend on the silica yield precisely matching expectations. For electronics manufacturing, customers chase batches with trace metals measured down to the part-per-billion, so tight quality control stays essential.
Manufacturers rely on direct reaction between silicon tetrachloride and ethanol, with careful removal of byproduct hydrogen chloride to ensure a clean end product. The process kicks off in reactors lined with materials that withstand both acid and organics. Temperature and pressure are watched closely, as the reaction’s exothermic punch can surprise operators. Small-scale labs might try simpler batch methods, but large factories automate the steps, saving product from picking up moisture on the way out. Repeated distillation and drying push out leftovers, giving a pure, storable liquid that ships well across continents.
TEOS stands out because it handles a wide range of functional groups. In hydrolysis, even small water traces unlock its ability to create silica gels or films. This feature powers sol-gel production, where researchers dial in the precise porosity, refractive index, and strength of coatings for lenses or circuit boards. Reacting TEOS with alkoxides or mixing it with titania, alumina, or other metal salts leads to hybrid materials that push the performance of electronics and membranes. Chemists often tinker with reaction speed by adding acid or base catalysts, opening new avenues for micro- or nanoscale silica particles. As TEOS meets more creative partners in the lab, the list of spinoff compounds keeps growing.
TEOS carries a heap of aliases in technical literature and on the shelf. Ethyl silicate, tetraethoxysilane, and silicon tetraethoxide point to the same stuff. Trade names crop up often in catalogs—for example, Fluka, Sigma TEOS, Aldrich Tetraethyl Silicate. Customs declarations sometimes use the UN number 1292, and regulatory paperwork prefers "tetraethyl orthosilicate" to dodge confusion with other alkoxysilanes. Buyers need to double-check SDS sheets because a synonym mismatch can trip up a project or trigger costly delays.
Working with TEOS calls for tight safety procedures. Liquid and vapors will irritate eyes and skin, so splash goggles and nitrile gloves become standard gear. Anyone handling large drums makes sure spill kits and eye wash stations are nearby. TEOS gives off ethanol and sometimes acidic fumes during storage or reaction, so proper ventilation protects lungs and keeps rooms below flammable vapor limits. Disposal involves neutralization and incineration under strict controls to stop environmental or health hits. Routine training drills and up-to-date chemical hygiene plans back up every operation—one careless step leads fast to accidents or regulatory trouble.
TEOS shines brightest in industrial and high-tech circles. The electronics industry values it for making silica insulators and layers in integrated circuits. Artists and conservators use it to reinforce stone or frescoes—turning delicate works into robust pieces able to battle humidity and pollution. Sealant makers count on its strong bonding with minerals for grouts and adhesives, while foundries use it for shell molds where shape accuracy runs critical. Lab-scale experiments lean on TEOS as a way to produce uniform silica particles, needed for chromatography columns or optical fibers. Even dental and orthopedic researchers tap it to help create custom-made ceramics and bioactive composites. The versatility grows every year and rarely gets boxed into one field for long.
For researchers, TEOS offers a toolkit for material design at the nanoscale. University labs routinely explore its sol-gel pathways to breed smarter membranes, filtration systems, or protective glasses. The hunt for cheaper, greener production methods led to ethanol recycling systems and solventless synthesis options. Some groups dive deep into doping TEOS-derived materials with metals or organic dyes, conjuring up new sensors or solar cell coatings. Collaborations across academia and industry push TEOS into territory no early chemist could predict—flexible silica electronics, energy-efficient windows, self-cleaning surfaces. Funding follows promising results, so the spotlight keeps on shifting as each new approach stirs up the field.
Toxicologists pay close attention to both TEOS itself and its byproducts. Large doses upset the nervous system and lungs if inhaled in an unprotected shop, though proper gear limits real-world risk. Skin contact burns, and people working with wet TEOS sometimes report dermatitis after a spill. Long-term animal studies show moderate toxicity, mainly linked to the ethanol produced during breakdown. Regulators flag TEOS as moderately hazardous, never a casual chemical, and tough rules govern air emission and occupational exposure. Water contamination worries researchers, but so far, well-run plants report only rare incidents, thanks to strict waste handling and on-site recycling. The overall message: respect the material, stick to tested safety rules, and ongoing monitoring cuts out surprises.
TEOS walks a promising path as the world leans into advanced materials. With industry demands for slimmer, tougher, and faster semiconductors, the need for ultra-pure silica never lets up. Environmental concerns push researchers to trim solvent use, recycle ethanol byproducts, and invent catalysts slashing energy bills. Biotechnology pioneers probe TEOS as a way to make next-gen implants, drug carriers, and diagnostic surfaces. Construction firms look for climate-friendly grouts and sealers where silica content ensures longer-lasting repairs. Every leap in green chemistry or digital technology gives TEOS a new reason to matter, driving fresh rounds of investment, patents, and real-world testing devices. For scientists and engineers, the challenge will be unlocking even more of its untapped potential—without losing sight of safety or environmental impact.
Tetraethyl orthosilicate, or TEOS, pops up in some pretty important places. Most people cross paths with its work through the glass on their smartphone, but TEOS never shows up by name on a box or display stand. I first heard about it back in college chemistry, where the professor explained how it helps to make specialty glass stronger. As someone who still drops their phone once a week, I’ve come to respect anything adding toughness to screens without making them thicker.
TEOS serves as a building block in the world of silicon-based chemistry. Chemists and engineers turn to it when they need to make glass coatings, sealants, or insulation materials. It enters the picture as a liquid. After some heating or exposure to moisture, it sheds its organic parts, leaving behind pure silica. This all takes place through a reaction called hydrolysis and condensation, which, after years spent in a research lab, has convinced me that messy liquids can birth the sturdiest glass.
Factories making microchips use TEOS for a whole different reason. Here, it's not about sheet glass at all. The material becomes the invisible spine inside the layers of circuits. During a process called chemical vapor deposition, TEOS converts into a gas that ends up as a wafer-thin film of silicon dioxide. This is how industry giants draw delicate insulators and create the scaffolding for billions of transistors. When I worked at a startup focused on solar panels, I learned that even clean tech chases purity and precision. TEOS offers a way to coat surfaces with exact layers, keeping electrical signals under control.
TEOS also finds work in construction. Concrete, while tough, lets water sneak in, and freezing or salty water eventually eats away at bridges and buildings. Construction crews sometimes mix solutions made from TEOS to add a protective glaze to surfaces. Sprayed or painted on, this coating reacts with moisture in the air to create a fine silica network. My cousin spent a summer in bridge maintenance, telling tales about rain finding its way through even small cracks, so it feels smart to use tricks from chemistry to ward off damage before it starts.
Labs also use TEOS for shaping biomedical devices and specialized coatings for implants. Silica-based materials formed this way tend not to irritate living tissue, which matters a lot if someone needs a replacement joint or a surgical sensor inside their body. Some teams have even used it to trap drugs inside porous glass, building timed-release capsules. After sitting in on a meeting between doctors and material scientists, the message felt clear: doctors and patients want more options that balance safety with performance—and TEOS helps build that bridge.
Dealing with TEOS has its risks. The liquid gives off vapors that can irritate the eyes and lungs. Labs and production lines work with solid ventilation rules and proper protective gear. It pays to handle it with respect. Factories using TEOS track their emissions and invest in scrubbers or closed systems, for both worker safety and good environmental stewardship.
Innovations using TEOS continue to push boundaries in construction, electronics, and medicine. This one compound started as a simple reagent, but through smart science and teamwork, it shapes the world around us—from cleaner bridges to sharper camera lenses.
Tetraethyl orthosilicate, often called TEOS, pops up in a lot of industries. People making glass, coatings, and some electronics deal with this stuff every day. If you’ve worked around solvents or chemical labs, TEOS goes from being a line on a safety data sheet to a very present reality, especially if none of your coworkers care much about ventilation or gloves. Health hazards can feel like background noise in busy workspaces, but TEOS doesn’t pull any punches.
Breathing in TEOS can irritate your nose, throat, and lungs. I’ve spent time around labs where the air starts to sting after a spill or a leak. TEOS evaporates quickly, adding fumes to the air. Over time, the irritation isn’t just a fleeting cough; it can set up a run of headaches, nausea, and dizziness. Long-term exposure increases risk of more serious respiratory problems. Splash some on your skin, and it burns. Eyes react with redness or pain, and that’s no small issue when precise work needs clear vision.
Data from the CDC and European Chemicals Agency echo the experiences people share on industry forums: TEOS vapor and liquid both harm tissues they contact. Studies in animal models even report damage to lungs after repeated inhalation exposure. The byproducts formed as TEOS breaks down, especially ethanol and silica, can add separate troubles. Ethanol vapors contribute to drowsiness, and amorphous silica dust brings its own set of lung risks if inhaled in large amounts, following similar patterns seen in silica-related occupational diseases.
The fix isn’t complicated theory. During an internship at a coatings lab, we used fume hoods and wore nitrile gloves religiously. People scoff at the gear until somebody gets a nasty cough or skin rash. Respirators and eye protection often feel superfluous, but the moment a TEOS bottle tips or leaks, anybody unprotected becomes an example. Ventilation, personal protective equipment, and strict storage rules form the backbone of practical safety. Information from OSHA and NIOSH points to the same: treat TEOS with as much respect as you would any aggressive solvent or reactive compound.
Signs on the wall only go so far. I’ve seen work environments where health protocols get drilled into new hires before any hands-on training. Weekly safety meetings reinforce what’s at stake, sharing actual stories of near-misses to keep lessons fresh. Realistically, health protection grows from habits and discipline, not wishful thinking. Proper labeling, storing TEOS away from open flames, and prepping staff for spills lead to fewer emergencies and less guesswork under stress.
TEOS brings clear benefits to industry, but the health costs become painfully obvious when shortcuts get taken. Listening to the science, human stories, and regulatory bodies means switching out luck for best practices. For anyone spending time in a lab, workshop, or factory with TEOS, knowledge and vigilance act as the strongest shields. TEOS isn’t the only tricky chemical out there, but it stands as a reminder: we can’t afford to get complacent with workplace health.
Anyone who’s spent much time around a lab or a manufacturing site knows there’s a world of difference between reading a chemical safety sheet and living with a chemical. Tetraethyl orthosilicate, or TEOS, makes plenty of products possible—from certain paints to electronics—but it doesn’t give you much room for mistakes. I’ve watched a careless spill go from “small mess” to emergency in less than a minute.
A drum of TEOS sitting in a corner doesn’t look menacing. The risk hides in its vapor and the way it reacts with moisture. TEOS fumes are a lung hazard, and contact with water starts a chemical reaction that releases ethanol. So if a container leaks or condensation builds up, the fumes might creep along the floor or build up in a closed room. A storage space for TEOS can’t just be any shelf in the shop. It’s got to be noticeably cool, well-ventilated, and dry—no leaks or drips from pipes overhead, no thunderstorms coming in through a cracked window.
Sealed metal drums or glass containers do the trick, but you can’t trust that seal forever. Over time, seals dry out; valves get brittle. I learned this after a valve failed in a not-so-old container, and I caught the sharp smell and shut the whole place down for the day. Regular checks on all containers count a lot more than buying the safest drum or a sturdy cabinet.
Opening or moving a can of TEOS calls for more than gloves and goggles. You need chemical-resistant gloves, splash goggles, and a real respirator—one filtered for organic vapors, not just a dust mask. Labs and shops both need fume hoods or local exhaust set-ups, since TEOS vapor doesn’t politely stay put. Trust me, those makeshift duct fans folks use just don’t do the job.
For busy labs, labeling seems simple, but confusion between solvents and similar looking colorless liquids has caused trouble more than once. We use bold, color-coded labels for TEOS, no exceptions, and keep incompatible chemicals—water, acids, and oxidizers—well away from the storage area. A separate spill kit, focused on solvents, stays within arm’s reach of every spot where TEOS might be used or stored.
Most incidents with TEOS come from people getting too comfortable—mixing containers, skipping checks, or rushing a transfer. Ethanol released in a humid room builds up without much warning. Storing TEOS below room temperature and away from sunlight slows down its natural breakdown. Cabinet doors get secured with signs for a reason. Fire extinguishers should cover more than just electrical fires; think class B resources for flammable liquid hazards.
Quality training and clear emergency plans have made the biggest difference in the safest shops I’ve known. Quick refresher sessions drive home what can go sideways. Folks remember those stories more than a list of chemical properties. Treating TEOS with the habit of checking, labeling, and venting has saved plenty of headaches—and maybe, someone’s lungs down the line.
Using less hazardous alternatives should be an ongoing discussion, but not every process offers that luxury. Whenever TEOS remains a must, tech like automated transfer systems and environmental sensors help. Electronic logs track when the cabinet opened and alert if the seal’s broken. That kind of early heads-up prevents small problems from becoming newsworthy accidents.
If knowledge is power, then sharing hands-on lessons can beat any warning label. People meet risk face to face in the small details—sturdy gear, sharp noses, and a willingness to stop the process long enough to check everything twice.
Anyone who’s ever worked around the lab bench, in industrial spaces, or even cleaning up after a spill has probably felt that gut-tightening moment when a chemical like tetraethyl orthosilicate (TEOS) turns up for disposal. TEOS isn’t just another bottle on the shelf—its sharp alcohol-like odor and quick hydrolysis remind you that this stuff is reactive and volatile. TEOS breaks down in contact with water and has links to lung and eye irritation, beyond flammability. You don’t shake off that knowledge, whether you’re in an R&D lab or a small-scale workshop.
The stories that stick often involve breathing in odd fumes or watching a beaker cloud over. TEOS hydrolyzes easily to ethanol and silica, which sounds harmless, but when this happens uncontrolled, toxic vapors, heat, and fine silica dust get released. This isn’t something an ordinary drain or trash bin can deal with. Every worker, student, or DIYer has likely seen the result of poor chemical management: corroded plumbing, environmental fines, and sick staff. Disposal mishaps with TEOS don’t go away quickly.
No shortage of regulations exists for chemical disposal. There’s nothing abstract or bureaucratic about keeping track of inventories and knowing your state’s hazardous waste rules. TEOS counts as a hazardous waste under US EPA’s Resource Conservation and Recovery Act (RCRA). Transporting or dumping it illegally can invite fines or worse. Local regulations guide every decision here, and turning over a sample to your environmental health office beats reading a government letter months later.
Chemists like me have learned the lesson by helping coordinate pickups and neutralized waste in community college stockrooms. Never combine old TEOS with regular solvents or toss it down the sink. Even empty containers need triple rinsing with a solvent that will break down traces safely; nobody wants to handle dry, crusted residue after months on the shelf. Seal everything in well-marked drums or containers. Each label should show the real contents and hazards, not mystery abbreviations. Only trained folks should touch a TEOS container once it leaves a research or production site.
Connecting with a licensed hazardous waste contractor often turns out best. These professionals bring tools and protective gear to pull off a collection safely. On-site neutralization sometimes looks easier, but unless chemical hoods, vacuum lines, and proper controls sit in place, you walk a dangerous line. Special incinerators designed for organosilicon compounds eat up TEOS—homebrew burning or open dumping just spreads risk to the next person and the water table. The Environmental Protection Agency tracks what contractors do; waste manifests don’t let anyone skip steps.
Even small users—art studios, consumer product formulators, or university labs—can use chemical collection events, sometimes free or low cost. A phone call sets up a safe handoff and brings peace of mind. I always tell colleagues that asking the "dumb" question about disposal is never a true mistake; one slip with TEOS can damage property and health in seconds.
The last bottle you use, not the first, sets the stage for environmental impact. Store only as much TEOS as truly needed. Rotate stockrooms, write clear logs, and encourage double-checks. Teach every new technician or student that finishing an experiment includes managing waste. Small habits—labeling, logging, calling a pro—matter for safety in labs, cleanrooms, and workshops across the country. Awareness reduces emergencies, legal headaches, and trips to the ER. That makes every bit of care worth it.
Tetraethyl Orthosilicate, often called TEOS, carries the chemical formula Si(OC2H5)4. One silicon atom partners up with four ethoxy groups. Structurally, this forms a central silicon atom bound to four oxygen atoms, each linked to an ethyl group. In the chemical world, such a composition opens the door to a range of reactions, thanks to those readily breakable Si–O–C bonds.
If you hold a bottle of TEOS, you’ll notice a colorless liquid. It exudes a somewhat ether-like odor, nothing sweet or harsh, just an unmistakable whiff that reminds me of time spent in college chemistry labs. It registers a boiling point around 168°C, which sits higher than most ordinary solvents but still manageable with standard lab equipment. With a density sitting close to 0.93 g/cm3 at room temperature, the liquid feels lighter than water.
Though it flows easily, TEOS suffers no affinity for water. Once it meets moisture, it starts kicking out ethanol and slowly hardens into a glassy, silica-like solid. That’s the root of why so many researchers, myself included, use it for sol-gel processes. Its low viscosity helps blend it with other chemicals, while its hydrolysis behavior creates thin films and coatings right on the workbench.
In the glass and ceramics community, TEOS spells opportunity. I’ve seen labs and factories reach for it to build up silica layers on microchips, strengthen optical fibers, or coat tiny particles for research. It stands out thanks to reliability and a knack for creating high-purity silicon dioxide at mild temperatures—impossible feats for traditional furnace methods. Since it’s liquid, it can get almost anywhere: onto a spinning glass disc, into a fiber preform, across a silicon wafer.
Whenever electronics shrink, insulation needs to thin out, and TEOS offers a smooth route. The purity pays off too, reducing unwanted ions that weaken insulation. Those working in heritage restoration use diluted TEOS to reinforce fragile sandstone or protect frescos from weathering, as I’ve witnessed on site visits—an IKEA-style fix for ancient relics.
Although TEOS remains handy, real-world use raises health and environmental hurdles. Breathing in vapor, especially across long shifts, can cause throat and lung irritation. It easily crosses glove membranes, so I keep thick nitrile gloves on hand and run fume hoods at full blast. Spilled drops evaporate slowly, so open windows and good air circulation matter. In the wrong hands, TEOS can cause skin rashes or eye harm.
Factories and universities manage the risks through sealed containers, spill kits, and tight training. Emergency eyewash stations sit close by in any responsible lab. The material reacts with water in drains, so neutralization steps show up in every disposal protocol. These safety layers let us use TEOS’s full potential without overshooting environmental safety boundaries.
Safer working habits work wonders. Switching to closed mixing systems and running automation mean less personal exposure. Seeking water-based precursors or greener organosilicates enters discussion every time supply chains falter or new regulations pop up. In my opinion, open conversations between chemists and environmental officers keep progress on track—because no exotic coating justifies an unsafe workplace or groundwater trouble.
Tetraethyl Orthosilicate brings advanced tech within reach but also demands careful handling. Balancing innovation with safety—this challenge will stick around as labs and industries keep searching for smarter materials.
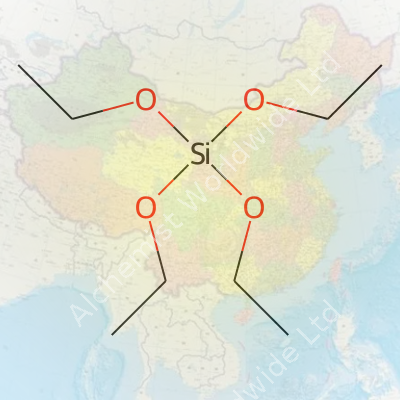
| Names | |
| Preferred IUPAC name | Tetraethoxysilane |
| Other names |
Tetraethoxysilane TEOS Ethyl silicate Silicic acid tetraethyl ester Tetraethyl silicate |
| Pronunciation | /ˌtɛtrəˈɛθaɪl ˌɔːrθəˈsɪlɪkeɪt/ |
| Identifiers | |
| CAS Number | 78-10-4 |
| 3D model (JSmol) | `$CCO[Si](OCC)(OCC)OCC` |
| Beilstein Reference | 1461001 |
| ChEBI | CHEBI:132870 |
| ChEMBL | CHEMBL135653 |
| ChemSpider | 6367 |
| DrugBank | DB11269 |
| ECHA InfoCard | 03f5c7c7-5cc7-4cdc-aafb-9c9a236fa794 |
| EC Number | 203-852-6 |
| Gmelin Reference | 25350 |
| KEGG | C08261 |
| MeSH | D013731 |
| PubChem CID | 6517 |
| RTECS number | VV7325000 |
| UNII | L5U3GWY7Q9 |
| UN number | UN1292 |
| Properties | |
| Chemical formula | C8H20O4Si |
| Molar mass | 208.33 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.933 g/cm3 |
| Solubility in water | Soluble (1.5 g/100 mL at 20 °C) |
| log P | 1.23 |
| Vapor pressure | 1 mmHg (at 25 °C) |
| Acidity (pKa) | Acidity (pKa): -2.6 |
| Magnetic susceptibility (χ) | -75.0e-6 cm³/mol |
| Refractive index (nD) | 1.382 |
| Viscosity | 2.5 mPa·s (20 °C) |
| Dipole moment | 2.53 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 354.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1467.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5642 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | V03AN01 |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H226, H332, H319, H335 |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338, P337+P313, P403+P233 |
| NFPA 704 (fire diamond) | 1-3-2 |
| Flash point | 41 °C (106 °F) |
| Autoignition temperature | 230 °C |
| Explosive limits | Explosive limits: 1.3–23% |
| Lethal dose or concentration | LD50 Oral Rat 6270 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 6270 mg/kg |
| NIOSH | ST3500000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Tetraethyl Orthosilicate: "Permissible exposure limit: 1 ppm (6 mg/m³) TWA |
| REL (Recommended) | 50 ppm |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Trimethyl orthosilicate Triethyl orthosilicate Tetramethyl orthosilicate Silicic acid Silicon dioxide |