
Long before the modern chemical plants took shape, folks experimented with silicates, trying to harness their potential in a variety of ways. Tetraethyl silicate entered the scene in the early 20th century, driven by both curiosity and industrial demand. As the world needed materials that could step up to the challenge of building and protecting, researchers poured time into understanding these quirky compounds. Back then, silica sol sources weren’t as refined as what we’ve got today. Chemists would recount how equipment corroded and fumes left the lab smelling sharp and unpleasant. The journey from lab curiosity to daily workhorse took decades, with newer purification techniques and better industrial controls leading the way. The compound's history isn’t something that felt distant—everyone working in materials science in the mid-20th century shared stories about silicate’s stubbornness and value in coatings, adhesives, and electronics.
Tetraethyl silicate kicks in as a clear, colorless liquid that gives off a slight, musty whiff. Chemists often call it TEOS, short for the much longer name. People in the business recognize it by other names—tetraethoxysilane, orthosilicic acid tetraethyl ester, and even just "ethyl silicate." Even as it spread through different sectors, workers and product users recognized TEOS because of both its feel and results: shiny protective layers, strong cross-linked structures, and a consistency that ordinary silanes couldn’t match. Specialty coatings, foundries needing strong molds, and even the circuit board industry all kept TEOS in stockrooms.
TEOS pours like any other solvent: light, easy to handle, and plenty volatile. Its boiling point comes in near 168°C, which means you’ll start noticing it evaporate faster than water once you get things warm. The liquid resists water at first, but if you add some acid or base, you’ll notice it hydrolyzes—breaking down into ethanol and silanol, then forming that tough silica glass everyone’s after. With its density hovering around 0.93 g/cm³ and a refractive index that’s been measured around 1.383, it’s clear enough to spot differences in composition if you know what to look for. Fire’s always a worry in the lab, since TEOS vapor catches alight if left near flames or sparks.
Over the years, I’ve seen bottles of TEOS labeled with varying levels of detail. The best producers spell out purity, moisture content, and hydrolyzable chlorine. Good manufacturers hit upwards of 99.5% purity on the main product, and the water content drops below 0.05%, since moisture spoils the fun in preparative chemistry and leads to unpredictable gels. Packaging matters too—aluminum, dark glass, or specialty polymer bottles make sense since sunlight or stray acids mess with the content. Proper hazard labeling jumps out at you: flammable liquid, irritant to eyes and respiratory tract, risk of delayed pulmonary effects. Regulations demand that, but the real motivation comes from hard-earned lab experience.
A good chunk of modern TEOS comes from the direct reaction of silicon tetrachloride with absolute ethanol. Anyone familiar with this reaction will recall the sharp, choking clouds of hydrogen chloride gas that billow off—you do it under a vent hood or not at all. Early attempts without modern equipment led to many ruined batches, stinging noses, and plenty of swearing. The mix boils under reflux, then distillers chase out impurities, scrub HCl, and dry the product. Some producers skip the tetrachloride, working up from elemental silicon with more elaborate alcoholysis, but the principle remains: drive off byproducts, watch the temperature, and avoid water.
TEOS finds itself at the heart of sol-gel chemistry. Add a few drops of acid, some water, and it springs into action—breaking bonds, then building new ones as siloxane chains take shape. The gel that forms winds up as high-purity silica glass after careful treatment. People have tinkered with TEOS for decades, loading it with dye molecules for colored coatings or weaving metal ions into the structure for unusual magnetic or electrical responses. Novel hybrid films appear all the time, showing off the flexibility of silica networks born from TEOS. Even simple hydrolysis, when done under the right conditions, brings out striking differences in surface texture and durability.
Chemists and industry veterans have never quite agreed on what to call this workhorse. Order catalogs list tetraethyl silicate, tetraethoxysilane, and orthosilicic acid tetraethyl ester—all the same stuff. Some suppliers sell it as “Ethyl Silicate 40” or other grades referencing dilution level or intended use. I’ve seen the same product on a shelf in Germany, labeled as TES, and labeled TEOS in a Chinese warehouse. It’s tough to confuse with anything else, given the smell and behavior, but the names trip up new workers every year.
Working around TEOS left me with no illusions about safety. The sharp fumes sting your throat and, if you don’t keep bottles closed, the risk of fire is always real. Spills clean up best with lots of ventilation and absorbent pads, and no one wants to find out how much vapor a spark can ignite. Industry safety data spells out the dangers: wear goggles, gloves, and a sturdy lab coat. Never pipet by mouth and always work inside a properly rated hood. Workers unlucky enough to inhale too much talk about coughing fits and watery eyes, and long-term studies urge caution—lung irritation doesn’t disappear overnight. Disposal takes care; it’s not wise to dump TEOS down a drain. I remember the time city authorities visited after an unintentional spill—cleanup teams arrived in bulky gear, and the paperwork seemed endless.
TEOS goes far beyond the academic laboratory. Construction teams rely on it for stone and concrete treatment. Architects rave about transparent coatings that keep marble steps looking sharp through rain, snow, and trampling feet. Foundries transform TEOS into investment casting molds—these molds hold up under wild temperature swings and help turn imagination into complex, sturdy parts for engines and turbines. The electronics field values its ability to deliver clean, glassy films on silicon wafers. Artists blend it into varnishes for a deep, lasting shine. If a field needs materials built to last, TEOS finds a home. I’ve seen it pop up in aerospace and energy research, a testament to its ability to bridge tradition and innovation.
Those who spend their days at the intersection of theory and practice keep pushing TEOS into new directions. Research teams tweak pH, solvent proportions, and temperature profiles to coax new properties out of old silica. One project I followed involved using TEOS as a foundation for self-cleaning glass in skyscrapers—a futuristic idea born from a century-old compound. Labs now chase silica aerogels with extreme porosity, insulating spaceships and bolstering oil recovery pipes. Military and medical scientists play with formulations, hoping to lock viruses out of surfaces or create safe, rigid implants. Curiosity and patience uncover each new use, often by chance or through months of persistent tweaking.
Tetraethyl silicate hasn’t always been given the scrutiny it deserves. Decades ago, most researchers treated it like any other nasty solvent, but as regulations tightened, toxicologists dug deeper. The compound’s acute toxicity doesn’t reach the danger levels of heavy metals, but vapor exposure irritates the lungs, and metabolites like ethanol pile up in the liver and blood. Chronic exposure causes respiratory trouble. Animal studies point to eye and lung inflammation, reinforcing what old hands have long observed in colleagues after careless handling. Personal experience and published research send a clear message: inhalation and skin contact must be minimized, protective barriers are not optional, and long-term studies still matter. Regulatory agencies follow emerging science, prompting tighter exposure and ventilation guidelines on shop floors and research labs alike.
Look at technology’s wild growth, and TEOS stands right on the launchpad for new achievements. The world wants materials that handle heat, cold, wear, and weather without falling apart, and TEOS stands ready as feedstock for next-generation glass, smart coatings, and safe medical implants. Sustainability’s on everyone’s mind, so green chemistry projects target renewable ethanol sources and improved purification. AI-driven optimization helps R&D teams find unexpected strengths in TEOS-derived films, boosting both performance and safety. From the race to build better batteries to efforts in 3D-printed silicates, the future for tetraethyl silicate looks busy. People who understand tradition keep the story moving forward, using time-tested knowledge and plenty of experimental grit to keep this classic compound working in new, better ways.
Tetraethyl silicate often gets attention in the world of raw chemical materials. In the lab, I remember seeing its strong smell and reading warnings telling me to keep the lid tight. Chemists work with it as a source of silica, which plays a key role in manufacturing. On the industrial scene, companies add tetraethyl silicate to coatings and paints. It doesn’t just sit there as a filler—it helps these coatings stick better to surfaces and keeps them from breaking down quickly. In situations where durability matters, like bridges or offshore rigs, engineers trust chemicals like this one to give long-term protection.
Glassmakers rely on silica compounds to control how their glass performs under heat. Tetraethyl silicate steps in as a way to prepare glass coatings or fibers. Factories use it to improve the finish and longevity of glassware people rely on every day, from chemical bottles to fiber-optic cables. The result isn’t just something that looks clear and smooth—the glass stands up to shocks, scratches, or high temperatures that come with heavy use. Ceramics benefit, too, because this compound helps shape textures and forms that make pieces stronger or give tiles the shine people want in kitchens and hospitals.
Anyone who’s opened a new electronics package might have seen a silica gel packet tucked inside. Those tiny beads grow out of chemistry involving tetraethyl silicate. Manufacturers use this compound to shape silica gels that keep shoes, gadgets, or food dry by absorbing moisture. It's not just about keeping boxes fresh—electronics get protection from the corrosion that humidity can bring.
Foundries and refractories use tetraethyl silicate to bind sand or other heat-resistant materials into shapes. I’ve seen molds made for complicated machine parts hold together under melting metal, thanks to this strong binder. If the binder fails, companies risk losing tools or time to repairs. Reliable molds keep costs down, which matters for both small shops and large plants.
Working with tetraethyl silicate comes with hazards. It releases alcohol vapors that can make people dizzy, and accidental spills trigger flammable fumes. My experience in the lab taught me that even a little carelessness can result in accidents—engineers use ventilation, fireproof storage, and safety gear as a baseline when handling it. In the manufacturing world, keeping employees safe means regular training, tight controls on ventilation, and spill response plans ready to go.
Studies from the National Institute for Occupational Safety and Health (NIOSH) show health risks like respiratory irritation or headaches at high exposure. Responsible companies set up air monitors and stick to safety limits. Regulators push for closed systems to lower the risk. Alternative binders or coatings have started to show up in research, as scientists look for safer substitutes that still work well in demanding industries.
People often overlook the role of industrial chemicals unless headlines bring hazards to light. I’ve noticed that smart choices in sourcing, handling, and disposal can make workplaces safer and the broader environment cleaner. Companies choosing tetraethyl silicate do so for its unique abilities, but they can’t ignore the need to protect their teams and neighbors. Real progress comes from better chemical management, smarter research, and keeping experienced workers involved in safety decisions.
Tetraethyl silicate shows up in a lot of industrial processes. It’s there in the production of coatings, adhesives, sealants, and sometimes as a bonding agent in ceramics. Chemists often call it TEOS or tetraethoxysilane. The substance itself, a colorless liquid with a sweet odor, doesn’t strike you as something especially threatening on sight. That can make it easy to forget the health risks hiding beneath that bland appearance.
Spending any real time reading material safety data sheets changes how you see chemicals like this one. The main issues come down to inhalation, skin, and eye contact. Tetraethyl silicate can irritate the lining of the nose, throat, and lungs. Breathing in the vapors brings on coughs, sore throats, even headaches or dizziness. Higher concentrations start to cause nausea, trouble breathing, and fits of coughing that just don’t quit. Some people, exposed for long periods, develop chronic respiratory complaints. I remember talking to a plant worker whose job involved frequent uncapping of drums. He described bouts of wheezing after his shift—clear evidence that the stuff isn’t just “irritating” in a mild sense.
It’s also rough on skin and eyes. Splashes sting instantly and can cause redness or even burns with enough exposure. The real risk, in my eyes, comes when you’re working in a rush, sweating, and you get careless handling containers. Even protective gloves sometimes fail if you work with the liquid for extended periods, especially if they’re not designed for organic compounds like this.
One thing we know: tetraethyl silicate doesn’t build up in human tissue the way some heavy metals or solvents do. But the lungs don’t appreciate repeated contact with vapors. Pulmonary edema—the buildup of fluid in the lungs—has been observed in acute overexposures. Some animal studies link repeated contact to kidney and liver damage. As someone who spent time in paint shops, stories of workers having trouble with kidney function years down the line changed the way I looked at labels.
What’s more, breaking down TEOS releases ethanol and silicon dioxide. Crystalline silica, the form of silicon dioxide that dusts off during some industrial grinding and finishing, gets flagged as a potential cancer hazard. Regulators pay close attention to workplaces using TEOS for that reason, not just for its direct effects but for what it becomes after use.
Proper ventilation matters. Not every workplace has up-to-date local exhaust, but the companies I’ve worked with that invest in it see fewer health complaints. Respirators, safety goggles, and gloves help—a lot—but using the right gloves for organic chemicals is key. For many jobs, vinyl or lightweight disposable gloves don’t cut it.
Clear training makes a difference. People who understand the hazard respect it more. I’ve seen workplaces where supervisors lay out the risks every morning, and workers not only use the gear but watch each other’s backs. That’s not just compliance for its own sake; that’s peace of mind.
Tetraethyl silicate does its job well in manufacturing, but that job comes with a cost. Health risks aren’t theoretical. With good ventilation, solid protective gear, and some real attention to safety training, factories manage those risks and keep workers healthier over the long haul.
Anyone who's worked with chemicals knows the headaches they can cause if left unchecked. Tetraethyl silicate, or TEOS as labs like to call it, often sits on shelves where safety takes a back seat to convenience. A careless approach can bring real trouble: fire risk, health scares, and even run-ins with the EPA. I remember a neighbor whose shop once reeked of solvents for weeks, thanks to a cracked storage drum. No one wants that mess on their hands.
TEOS has a nasty side. This liquid starts to break down with moisture and sends off flammable vapors, making it more than just a paperwork task. The National Fire Protection Association gives it a flammability rating of 2 out of 4. One spark from static or a dropped tool can turn a simple spill into a crisis. Even for small labs or workshops, this isn’t something to brush off.
Skin contact brings irritation, inhaling its fumes hammers your lungs, and swallowing it can mean an emergency room visit. The Centers for Disease Control and Prevention backs this up. If a leak happens, those vapors will not just bother your nose — they can do real damage after minutes. So, safety isn’t just about saving product but protecting people on the ground.
People can keep things safer by following three main pillars: location, container, and regular checks. For starters, TEOS demands a cool, dry spot. Humidity sets off the breakdown process. My own experience with a poorly sealed drum in a humid basement taught me the hard way—the stench clung for days and the product wound up wasted.
Metal drums lined with plastic or high-density polyethylene jugs work well, provided the seals fit snugly. I never skimp on checking gaskets or closures. A cracked cap invites leaks or air inside. Keeping chemicals in glass isn’t smart since glass shatters if dropped. Plastic containers, made for solvent resistance, stay my go-to for smaller batches.
Labeling matters too. I put the product’s name, the hazard class, and the date received right on the front. Clear labels save time during emergencies and keep everyone aware of what’s inside. There’s no such thing as redundant safety info when a quick glance makes the difference.
Storing TEOS far from heat or sparks beats any fire extinguisher. Fume hoods run in my shop wherever solvents rest for long periods. Static buildup crops up in dry climates, so using grounded shelves or a grounded drum cradle helps keep accidental sparks off the table.
TEOS belongs alongside other flammable liquids—not with acids, bases, or oxidizers that could kick off a chain reaction. Segregation in the storage room keeps one mistake from turning into a full-blown incident.
Anyone given the key to a chemical store room, whether a part-time intern or a company owner, should walk through the safety basics. Training covers everything from spill containment to quick cleanups and knowing who to call for backup. OSHA points out that clear guidelines cut down on missteps and keep the workplace calm.
Routine inspections catch issues before they snowball. I check my storage area every week—sure, it takes time, but it beats scrambling to deal with a leak or failing a surprise inspection.
Treating tetraethyl silicate storage like an afterthought only raises the odds for disaster at work or at home. Paying attention to the containers, the climate, and the people around builds a layer of protection. Real safety grows from good habits, regular checks, and a healthy respect for what’s at stake. A few minutes up front beats hours of regret after the fact.
Tetraethyl silicate pops up in industries where coatings, adhesives, and high-performance glass come to life. It sounds far removed from typical household chemicals, but the risks feel all too real for workers and anyone in close proximity. Its chemical nature turns simple mistakes into more serious consequences if you cut corners on safety.
Breathing in tetraethyl silicate doesn’t cause a tickle or simple sore throat. The released vapor has a knack for irritating the nose, throat, and lungs. Enough exposure builds up to headaches, dizziness, or nausea. Those who have spent hours around this compound, sometimes skipping mask protection for “just five minutes,” know that these symptoms hit fast and hard. Repeated skin contact has a habit of drying hands and setting off nasty dermatitis that lingers long after the shift ends.
Lab studies point out real dangers. Both the US CDC and European Chemicals Agency flag tetraethyl silicate as hazardous if inhaled, swallowed, or spilled on skin. Severe eye contact cases—even brief—can lead to blurred vision and pain. Even small spills, left unattended, spread into the air and threaten everyone in the vicinity. These aren’t one-off cases.
Working with tetraethyl silicate always calls for proper PPE. I remember rushing through experiments once in a glove that had a small tear. The resulting rash stuck with me for weeks and served as a steady reminder that chemical protection equipment isn’t just about ticking boxes. The right gloves—nitrile or butyl rubber—block direct contact. Tightly fitting goggles stop invisible mists from landing in the eyes, which is a nightmare scenario nobody wants to face.
Ventilation stands as a frontline defense. If you walk into a room and smell a sharp, sweet odor, you’re likely inhaling low levels that build up risk over time. Chemical fume hoods or extraction fans make a real difference, not just as fancy installations, but as required barriers to serious health problems.
It’s easy to get comfortable on the job, following muscle memory and hoping for smooth sailing. Tetraethyl silicate doesn’t forgive shortcuts. Training that actually simulates spills and emergency situations turns people from bystanders into quick responders. Having real-life practice using eyewash stations and emergency showers makes those precious first seconds count, especially when someone splashes material on their face or arms.
Storage also decides a lot about long-term safety. Tetraethyl silicate reacts with water and acids, so careless placement near plumbing or strong chemicals sets up the perfect storm for leaks or hazardous reactions. Storing the chemical in clearly labeled, tightly sealed containers away from heat or flame, and keeping up regular checks, takes guesswork and stress out of day-to-day operations.
Nobody expects accidents until they happen. Consistent routines—checking gloves for holes, double-checking ventilation, briefing teams before new projects—make the difference between safe handling and chaos. I’ve seen places where colleagues quietly look out for each other, reminding someone to swap out stained gloves or flagging a vent that’s lost power. That hands-on attitude does more to build safety than any laminated poster or annual seminar.
Employers have a duty, and so do workers, to keep up these habits. Quick access to material safety data sheets, regular disaster drills, and involvement from everyone—rookie and veteran alike—keeps risks down and morale up in workplaces dealing with tetraethyl silicate.
Safety around tetraethyl silicate doesn’t spring from luck. It lives in the actions, routines, and decisions people make each day.
Tetraethyl silicate pops up in different places—think about coatings, paints, and even the process behind some glass manufacturing. Most folks outside a lab don’t give it much thought. If you ever handled the liquid or read the label, one big question always rises up: can you just mix it with water? It sounds like an everyday test, but this single issue matters a lot for anyone managing chemicals at work or storage at home.
Tetraethyl silicate (often called TEOS in labs) doesn’t play well with water. Scientists have tested and watched; the compound stays almost entirely separate. Chemically, it’s not surprising. The structure of tetraethyl silicate puts four ethoxy groups around a silicon atom. Water, being the classic polar liquid, doesn’t hook up with those groups very easily. What actually happens? Pour TEOS into water and you’ll just see two layers, not a dissolved liquid.
I learned in school labs—messy, sometimes under-ventilated places—how complicated simple mixing can get. When a classmate once tried to dump a sample of TEOS into a water bucket, expecting it to wash away quickly, we ended up with a sticky mess clinging to glass and plastic. That sticky residue created more cleaning, more exposure risk, and always a little chaos in the classroom. I haven’t forgotten how tricky chemical properties sneak up on you.
Companies dealing with TEOS pay attention because solubility affects everything from cleanup protocols to accident prevention. Poor water solubility means you can’t just flush a spill with water and call it safe. Instead, you might spread the mess or, worse, trigger unwanted chemical reactions. For safety teams, this is a headache because it pushes for specialized spill kits, thorough training, and constant reminders never to take shortcuts.
It doesn’t stop with labs or factories. Even in a hobbyist’s garage, misunderstanding the chemical’s behavior risks personal harm. TEOS can react with moisture (slowly at room temperature) to release ethanol, which isn’t hazardous in small amounts but changes the safety plan if you spill a lot. Disposal rules grow stricter if you can’t dilute something in water. Water companies and treatment plants would have a tough time contaminant-catching if washing down sinks became the norm. Environmental regulations actually rely on toughness with poorly soluble chemicals to keep drinking water clean and workers healthy.
Getting people the right information should land at the top of the list. Training—hands-on, not just reading sheets on a wall—helps everyone see what happens if you mix TEOS with water. You don’t want guesswork guiding these choices. Marking storage containers, clarifying cleanup steps, and using personal protective equipment remains essential. Chemistry doesn’t allow much room for error.
If you work with or around tetraethyl silicate, lean on product safety data sheets updated by experts, not just last year’s guide. Facility managers can aim for on-site demonstrations, so staff understand the results of a simple pour, not just diagrams or theory. Engineering controls, such as sealed containers, ventilation, and quick access to the right absorbent materials, pay off fast if something spills. Work culture grows stronger when people feel trusted and informed, and that never happens by pretending safety is common sense when it really comes down to knowing the facts.
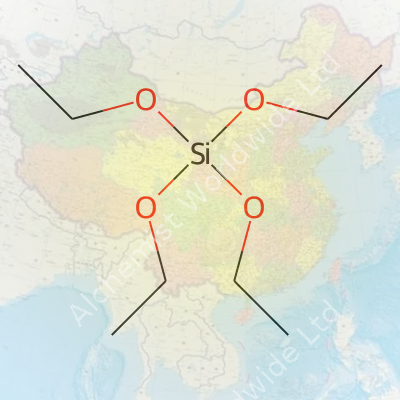
| Names | |
| Preferred IUPAC name | Tetraethoxysilane |
| Other names |
Tetraethoxysilane TEOS Ethyl silicate Silicic acid tetraethyl ester Tetraethyl orthosilicate |
| Pronunciation | /ˌtɛtrəˈɛθɪl sɪˈlɪkeɪt/ |
| Identifiers | |
| CAS Number | 78-10-4 |
| Beilstein Reference | Beilstein 969073 |
| ChEBI | CHEBI:30561 |
| ChEMBL | CHEMBL154197 |
| ChemSpider | 6922 |
| DrugBank | DB13924 |
| ECHA InfoCard | ECHA InfoCard: 027-001-00-9 |
| EC Number | 203-852-3 |
| Gmelin Reference | 60379 |
| KEGG | C06521 |
| MeSH | D013737 |
| PubChem CID | 6629 |
| RTECS number | VV9275000 |
| UNII | 4P6I9D4BDY |
| UN number | UN1292 |
| Properties | |
| Chemical formula | C8H20O4Si |
| Molar mass | 208.33 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.933 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.45 |
| Vapor pressure | 1 mmHg (at 20 °C) |
| Acidity (pKa) | 10.7 |
| Basicity (pKb) | Product Tetraethyl Silicate has a pKb (Basicity) of 21.56 |
| Magnetic susceptibility (χ) | −54×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.381 |
| Viscosity | 3-5 mPa·s (25°C) |
| Dipole moment | 0.04 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 272.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1497.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6161.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS02, GHS07, GHS08 |
| Signal word | Danger |
| Hazard statements | H226, H332, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P304+P340, P312 |
| NFPA 704 (fire diamond) | 1-2-0- |
| Flash point | 45 °C |
| Autoignition temperature | 250 °C |
| Explosive limits | Explosive limits: 1.5–48% |
| Lethal dose or concentration | LD50 oral rat 6270 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat 6270 mg/kg |
| NIOSH | WX8225000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Tetraethyl Silicate: "PEL = 10 ppm (85 mg/m3) (OSHA) |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | 700 ppm |
| Related compounds | |
| Related compounds |
Trimethyl orthosilicate Tetramethyl orthosilicate Tetra-n-propyl orthosilicate Tetra-n-butyl orthosilicate |