
Chemists have been tinkering with silanes for over a century, always trying to build on what early pioneers like Alfred Stock started in the early 1900s. Tetrakis(2-methoxyethoxy)silane didn’t appear until the latter half of the 20th century, riding a wave of organosilicon chemistry focused on practical uses in coatings and electronics. Researchers wanted more functional, stable silanes for sol-gel processes and surface modification. This specific compound answered that call, bringing improved handling and reactivity for chemists working out solutions for everything from glass modification to polymer synthesis. Experience in the lab shows that newer silanes often replace older options by cutting down on side reactions and health worries, marking a logical step from the haphazard organosilicon explorations of a few decades ago to more controlled synthesis today.
Tetrakis(2-methoxyethoxy)silane gives chemists a practical option for transferring silicon into organic or hybrid environments, especially where custom surface properties matter. It often comes as a colorless or light-yellow liquid, packaged carefully to handle moisture sensitivity. The appeal comes from its mix of four 2-methoxyethoxy groups, which boost solubility and compatibility with both organic solvents and certain acrylic or urethane systems. Industries lean toward it for tasks that call for durable, flexible coatings or highly engineered surfaces, where off-the-shelf silanes can’t quite meet demanding specs. People often turn to this silane because they want direct results in applications that include improved adhesion and minimized phase separation.
Bringing practical knowledge from the lab, tetrakis(2-methoxyethoxy)silane stands out for a boiling point around 290°C under normal pressure, though it starts to decompose if heated too aggressively. Its density hovers near 1.05 g/cm³, which makes measuring and transferring simple for researchers accustomed to organic liquids. Moisture quickly cracks it open, splitting off 2-methoxyethanol and producing silanols, so folks working with it keep it away from water and humid air. The large, flexible side chains built around the silicon atom let this molecule dissolve in many organic solvents but show low miscibility with water. Handling it over the bench always reminds you that even small humidity sneaks in and can change the character quickly.
Manufacturers who supply tetrakis(2-methoxyethoxy)silane usually report purity above 97%, with water contents under 0.1%. The typical CAS number is 18765-38-3, and standardized labeling per GHS guidelines warns about skin and respiratory irritation. Labels list boiling point, density, and storage advice—store under nitrogen, keep away from light, and stick to glass, stainless steel, or certain plastics. Any shipping document also highlights the liquid’s flammability, so chemical buyers check the paperwork for UN numbers and hazard pictograms. I’ve learned to look for fresh production dates since hydrolysis, even from slow leaks, can gum up a bottle over time.
Nobody gets this silane without thoughtful synthesis. The go-to method starts from silicon tetrachloride and 2-methoxyethanol, using a base such as triethylamine to mop up hydrochloric acid as the ether groups latch onto the silicon core. The process needs dry conditions and careful temperature control, or else competing side products pile up. Fractional distillation separates pure product from unreacted alcohols and byproducts. This isn’t something to whip up in a high school classroom; it takes good glassware, reliable vacuum systems, and solid chemical safety practice. Many in research and industry still buy it pre-made rather than synthesizing in-house, avoiding waste and improving reliability in their results.
In seeing how this compound behaves, you notice right away that its reactivity centers on the silicon-oxygen bonds. Exposed to moisture, it hydrolyzes fast—making it a favorite for sol-gel processing where controlled silicon oxide networks form. The 2-methoxyethoxy groups slowly release under heat or acid, opening up new reactivity paths such as forming crosslinked siloxane structures. Surface chemists use it for anchoring organic layers on glass or metal oxides, enhancing wear resistance, or improving wettability. Other modifications often swap these ether side chains with different alcohols to tweak performance. The versatility comes from tuning the chain length or introducing new functionalities for later coupling steps. Most who research surface modification eventually run into this compound or its close cousins, since it’s a simple way to introduce tailored chemical groups into a material.
On chemical order forms or product sheets, tetrakis(2-methoxyethoxy)silane appears under several names. Some know it as Tetrakis(2-methoxyethoxy)silicon, or its German trade names like Tetrakis(2-Methoxy-äthoxy)Silicium. Its CAS number, 18765-38-3, often stands out in catalogs. Commercial options might add “purified” or “high purity” in their descriptions. I’ve even seen manufacturers shorten it to 2-MEES or TMES for brevity, especially in research papers focused on sol-gel chemistry or advanced materials.
Safe handling practices matter with this compound. Anyone using it in an industrial or lab setting wears goggles and gloves, and avoids splashing it around. Inhalation irritates airways and skin contact stings, largely because of the slow hydrolysis and byproduct release. Storage under nitrogen or argon prevents dangerous buildup of acidic alcohols or pressure. Facilities use explosion-proof equipment for bulk handling, since vapors pose fire risks near ignition sources. Spills need drying agents such as silica gel to pick up the liquid and stop surface corrosion. Trainings stress the importance of monitoring air quality, especially where open containers might release volatile organics—OSHA or European guidelines push for regular ventilation checks. Real-world incidents show that hurrying or skipping steps can turn a routine transfer into a serious incident quickly.
Modern technology leans on specialized materials, and tetrakis(2-methoxyethoxy)silane finds heavy use in surface coating, electronics, and composite production. Sol-gel firms use it as a silicon source for glassy films at low temperatures. Paint manufacturers blend it into primers and surface treatments, boosting scratch resistance and hydrophobicity. Printed circuit board manufacturers use it for insulating layers, where reliability at high frequency comes first. Laboratory researchers see it as a good link between inorganic surfaces and polymer matrices, helping adhesives bite into glass or metal. Memory from time in the lab sticks: using the right silane often makes or breaks whether a coating actually adheres through aging tests or exposure to weather.
Silanes rarely stand still—teams around the globe try to push boundaries with small tweaks to function. Tetrakis(2-methoxyethoxy)silane appears in studies on nanomaterial synthesis, where the precise structure of the ether arms directs the growth of ordered arrays or nanoporous silica. Polymer chemists test out new formulations where these silanes boost flexibility or resistance to cracking. Intellectual property filings reveal an uptick in patents on hybrid interfaces, trying to exploit the balance of hydrophilic and hydrophobic elements in a single molecule. Many university groups choose to compare this silane side-by-side with older alkoxysilanes, looking at durability or barrier performance. From years of reading and collaborating with surface scientists, it’s clear they view organosilanes not as mere reagents but as tools for building reliable, performance-driven coatings and films.
Concern for toxicity grows with increased use, so toxicologists run animal and cell-line assays to catch harmful effects before these compounds hit the market in bulk. Tests have shown that hydrolysis byproducts such as 2-methoxyethanol deserve special caution, since this ether can cause reproductive and developmental problems. Regulatory agencies in Europe and North America monitor workplace exposure levels and encourage substitution with less risky compounds where practical. Chronic skin contact or inhalation could trigger nerve or liver problems, and chemical hygiene plans set strict limits for airborne concentrations. The trend in safety research points toward engineering controls and personal protective equipment as ways to keep operators in the clear. I’ve seen first-hand the focus on risk assessments that look beyond the silane itself to include breakdown products, which sometimes slip through standard detection screens until more rigorous testing is adopted.
The next chapter for tetrakis(2-methoxyethoxy)silane likely includes broader use in energy materials, advanced coatings, and even biomedical devices where biocompatibility and tailored surfaces matter. Researchers look for hybrid materials combining silicon’s backbone strength with the versatile chemistry of organics, and they see this silane as a near-perfect starting point for building such structures. Economic and environmental pressures will drive synthesis toward greener routes and safer ether combinations, spurred by persistent questions about toxicity. Teams in industry focus on refining performance—say, getting longer-lasting protective finishes or improved adhesion across difficult substrates like plastics and ceramics. Years of experience in both research and industry show that once a silane like this earns trust in high-value applications, new uses follow as engineers and scientists build confidence, scale up, and share hard-won lessons across fields.
Tetrakis(2-Methoxyethoxy)Silane wears the chemical formula Si(OCH2CH2OCH3)4. Underneath these symbols, you get a silicon atom surrounded by four 2-methoxyethoxy groups. Each of those branches gives the molecule a certain flexibility in different chemical environments, making it more than a spectator in the lab or factory floor.
You won’t just see this molecule listed in a dusty chemical catalogue. Tetrakis(2-Methoxyethoxy)Silane plays an important part in electronics, coatings, and specialty glass. Land in a technical meeting about semiconductors, and someone probably mentions these organosilicon compounds for reasons tied to their volatility and reactivity. See, people value their purity and the way they can deposit thin, even layers of silicon-based films.
Science doesn’t move forward through catchy acronyms alone. Experience in a materials lab or process engineering department shows how small tweaks in chemistry can raise reliability and cut production headaches. Tetrakis(2-Methoxyethoxy)Silane offers that sort of precision.
Every chemist knows a little snag can throw off months of planning. Impurities in precursors like Tetrakis(2-Methoxyethoxy)Silane can affect the final product, leading to costly recalls or device failures. Silicon sources can carry water or oxidize, and even tiny amounts of contamination can impact high-performance microchips or coatings.
Those who have worked around specialty chemicals recognize that meticulous handling, storage at the right temperature, and careful monitoring of moisture change the game. Sometimes, a trace impurity goes unnoticed until a sudden blip in yield or a subtle change in physical properties tells you something’s slipped. There's nothing abstract about time lost on diagnostics or unhappy meetings with quality control.
People expect more from industry, both in terms of performance and environmental safety. Safe handling guidelines, transparent supply chains, and nods to sustainability matter just as much as a molecule’s reactivity. Tetrakis(2-Methoxyethoxy)Silane, used wisely, supports advances in more energy-efficient electronics or cleaner chemical processes. Manufacturing plants and R&D labs tackle real risks around inhalation or flammability by enforcing training and using smart containment systems.
Still, no chemical story stays the same. Colleagues in research push for methods that use less hazardous reagents or recycle waste products, showing innovation walks hand-in-hand with caution. Where Tetrakis(2-Methoxyethoxy)Silane fits, demand for real traceability and compliance with regulatory standards keeps everyone honest.
Experience shows that better monitoring catches contamination sooner, protecting both products and people. Investing in analytical tools—like high-resolution mass spectrometry—has a direct impact on efficiency. Regular training grounds staff in practical safety skills, which cannot come from manuals alone.
Working with specialty silanes such as Tetrakis(2-Methoxyethoxy)Silane spotlights the connection between quality control and innovation. Hard-earned lessons shared among chemists, engineers, and safety managers provide the real backbone of progress in any field using this unique compound.
Tetrakis(2-Methoxyethoxy)Silane doesn’t pop up in cocktail conversations, but it definitely helps shape our world behind the scenes. Most folks working in electronics or surface treatments will run into it sooner or later. The compound goes by its nickname, TMOS, in some circles. If you take a look around modern industry, you’ll spot its fingerprints – from high-end electronics to anti-fog coatings on goggles. There’s real reason experts turn to it for specialized jobs.
Clean room teams in chip factories count on compounds that won’t gum up the works. Tetrakis(2-Methoxyethoxy)Silane gives them an even, predictable silicon source for processes like chemical vapor deposition. This method is what lets microchips grow their wafer-thin layers. Gadgets keep shrinking, and the demands on purity and control keep climbing. By providing a stable silicon source that resists early reactivity with water, this compound delivers reliable results, support tight tolerances, and keeps waste in check. With the global semiconductor market projected to top $1 trillion before 2030, according to McKinsey, the need for chemicals meeting these standards only grows.
Anyone who's ever wiped their glasses and wished the fog would vanish, owes something to the chemistry behind advanced coatings. Tetrakis(2-Methoxyethoxy)Silane plays a key role in the primer step of making anti-fog, scratch-resistant, or water-repellent coatings. A thin, invisible layer can anchor other treatment chemicals better than older methods. Lenses stay clear, windshields shed rain, and display screens stand up to fingerprints. Studies published in journals like Surface & Coatings Technology highlight the efficiency boost from using organosilane molecules with flexible side chains, just like the methoxyethoxy groups here.
Tetrakis(2-Methoxyethoxy)Silane pops up in creating sol-gel materials. These start as liquids but end up as solid glass or ceramics after some careful processing. The compound gives researchers a way to tune the flexibility or toughness of the result — key for lightweight aircraft parts or next-gen insulation. The aerospace, construction, and advanced manufacturing spaces sink millions of dollars every year into R&D hoping to make lighter, stronger, and smarter building blocks. According to reports from MarketsandMarkets, the global sol-gel market is growing at over 10% a year, driven by demand for better insulators and specialty glass.
Tackling a chemical like this comes with real headaches. Big costs and strict safety protocols show up everywhere. Even a little moisture can set off unwanted reactions, which means companies pour money into sealed systems and careful storage. Green chemistry principles call for safer, less toxic replacements. Some labs work on alternatives but haven’t found one that matches the performance edge. Safer process designs, better worker training, and push for recycling unused compound all help cut the risks. Academic groups and large manufacturers both keep hunting for breakthroughs — that’s often how the next big leap happens in specialty chemicals.
As the tools and toys that rely on silicon get smarter, manufacturers lean even harder on compounds that deliver at the molecular level. Tetrakis(2-Methoxyethoxy)Silane holds a quiet but pivotal spot in that effort. The future likely sees cleaner, safer, and perhaps even bio-based alternatives, but for now, this compound’s real-world impact is clear, especially in electronics, coatings, and advanced materials.
Few things demand more respect than chemicals with risks attached. Keeping a product stable and safe depends on basic, clear-sighted habits in storage and handling. Shelving, organization, labeling — each step matters, and trouble often starts with small oversights.
Heat triggers reactions in many products. Even a summer day inside a poorly ventilated warehouse raises risk. Manufacturer recommendations guide temperature limits for a reason. I’ve seen drums of chemicals expand, vent or leak on hot days, just from sitting near sunny loading doors. Cool, shaded, consistent conditions cut these risks. Airflow lowers hot spots and condensation. Indoor storage away from direct sun, and away from heat sources like generators or boilers, keeps products within their safe range.
Damp air, leaking roofs, or spilled liquids inside a facility spell trouble. Moisture can start reactions or break down packaging. Once, a small water leak in a supply room led to damaged bags and odors that cost time and money to fix. Absorbent products swell, cakes form, and powders clump. Using pallets or shelving gives space for air to circulate, and prevents bags or containers from sitting in puddles or on floors.
Cleanliness sounds simple, but stray dust or residue creeps up. Dedicated storage, regular cleaning, immediate spill response—these habits keep environments safe. Segregating chemicals that can react or contaminate each other helps, especially with acids, oxidizers, or flammables.
Labels save lives and protect workers. Original labels tell the story: names, hazard warnings, and emergency measures. If a label fades, gets torn, or falls off, replace it with the same critical details. During a plant visit, I watched a supervisor catch a minor mix-up—labels had peeled off during re-packaging. Quick action prevented misplacement and mistakes that could have escalated.
It’s not just about legal compliance. Training every team member to check and double-check products before moving, using, or mixing them supports a safe environment. Sharing clear instructions on what to avoid—extremes in heat, mixing with incompatible products, or storing by open drains—gives teams practical know-how.
Experience taught me that consistent PPE use slashes accidents. Long pants, gloves, masks, and goggles aren’t optional; the right fit and material matter. Use scoops, tongs, or pumps for dispensing where possible. Direct contact, even once, can bring rashes, breathing problems, or worse.
Ready access to spill kits, eye wash stations, and emergency showers shows respect for danger—not just a regulatory checkbox. Rushed transfers or careless handling cause more trouble than careful, deliberate action ever will.
Customers count on clean, safe products. Improper storage can mean poor performance, safety failures, or ruined stock. Word spreads fast in any supply chain, and companies can lose credibility over careless storage. Employees stay safer, too, when rules aren’t skipped and shortcuts don’t become habits.
Trust grows from everyday attention: good habits, clear labels, routine checks, clean storage, proper PPE—all these build a system with no weak links. Word to the wise: fix small issues before they grow into real problems.
Tetrakis(2-methoxyethoxy)silane shows up often in laboratories and production floors. Chemists use it to make advanced materials, mostly because it helps link chemicals together. The people handling it want to know: does this compound threaten their health? Will it seep quietly into soil or water and cause trouble outside the lab?
If you work with silanes, you probably remember the harsh odors and strong irritation they can cause. Tetrakis(2-methoxyethoxy)silane, with its ether groups, can irritate lungs, skin, and eyes if spilled or vaporized. Accidental splashes mean burning sensations or worse. I have seen coworkers cough and rub their eyes after exposure to low levels—personal protection matters. Breathing in vapors, even at levels below the legal limit, can leave people with throat discomfort and headaches.
Using gloves, goggles, and proper ventilation does help reduce risk. OSHA and similar agencies stress the need for tight controls because silane molecules often pass through latex and standard rubber. Chemical-resistant gloves such as nitrile or butyl rubber make a difference. Safety teams run regular air samples to catch rising levels of vapors, not just for this silane but for similar ethers and organics. Taking shortcuts here only leads to long-term health complaints from staff.
If someone swallows even a small amount, stomach cramps and vomiting follow. Emergency teams recommend quick rinsing of eyes, immediate removal from the exposure zone, and seeking medical help for inhalation or ingestion. Emergency room nurses have shared stories of not recognizing the danger at first, only to see escalating symptoms once a patient explains the chemical’s name. Fast action with accurate information matters for the best outcomes.
Factories do not run in a vacuum. Spillage, rainwater runoff, or accidents can push compounds like tetrakis(2-methoxyethoxy)silane into local drain systems. Once there, the compound might break down slowly depending on the environment. In waterlogged soils or rivers, decomposition speeds up, but fragments can linger, threatening aquatic bugs and small fish.
When manufacturing controls slip, these silanes may attach themselves to organic matter and drift through groundwater. Ecosystem biologists worry because persistent organics accumulate over time. Even if they break down, byproducts sometimes create new problems. For this reason, companies that use or ship tetrakis(2-methoxyethoxy)silane must invest in spill-containment, leak detection, and proper waste disposal.
On-the-ground workers trust their companies to prioritize safety. Engineers design closed systems and sealed storage containers for silane-based chemicals. Labs invest in air monitoring sensors and fire suppression because these compounds can ignite. Training helps, but culture matters more: if a team feels rushed, mistakes creep in, no matter the safeguards listed in manuals.
EHS professionals—people with boots on the ground—walk the storage yards, looking for leaks or pooling chemicals after storms. They collect samples and keep records, not just to meet regulations but to show neighbors and staff they care about the local creek and air. A spill or accident gets investigated, not hidden.
Alternative chemistries exist for some applications, but switching can require months or years of trial and error. Where substitution lags, mitigation stays crucial. I have watched chemical plants add vapor scrubbers and double-walled tanks, even when not explicitly required, to keep silanes where they belong.
Using hazardous chemicals like tetrakis(2-methoxyethoxy)silane brings responsibility. Transparency in incident reporting, robust protective equipment, and strong environmental monitoring form the backbone of true risk management. In the end, asking tough questions, listening to staff, and keeping up with research will do more for safety than any checklist or compliance audit.
Working with organosilicon chemicals like Tetrakis(2-Methoxyethoxy)Silane means playing by a different set of rules compared to things most folks keep under the sink. The chemical formula doesn’t only look complicated, the molecule itself brings a lot of quirks. Anyone handling it in a lab or on the factory floor isn’t just ticking boxes—they’re blocking trouble before it starts.
Anyone who’s pulled a forgotten reagent from the back of a chemical cabinet knows the mix of hope and worry. Silanes in general don’t handle moisture well, and Tetrakis(2-Methoxyethoxy)Silane is no exception. Most suppliers give this material a shelf life of 12 to 24 months, but that window narrows fast if the bottle ends up near heat or drafty windows.
A short shelf life means less flexibility for research teams or manufacturing operations. Timing orders and using up stock before degradation affects purity gets tricky. Degraded silane doesn’t just lose performance—it can bring unpredictable byproducts. For anyone working in electronics or advanced coatings, that unpredictability can mean botched batches and wasted resources.
Organosilicon compounds like this silane break down faster at higher temperatures. Storage targets sit between 2°C and 8°C—standard refrigerator temperatures. Some folks stretch to “room temperature” ranges, but every degree over 25°C invites headaches. Even a closet in an office can spike in the summer, and after enough days above the mark, stability takes a hit.
In one case I remember, working late in a university setting, our team learned the tough way that just a week in an overheated storage area turned valuable material into a sticky mess. The supplier confirmed the stability limits afterward—always refrigerate, keep the cap tight, and go through the batch inside a year. Simple advice, but that oversight set us back, burned the budget, and wasted hours of follow-up analyses.
A “close enough” storage system often looks affordable but ends up costing more in discarded material and extra monitoring. Moisture causes hydrolysis, knocking out the silane groups that make this compound useful in the first place. Even trace humidity inside a storage bottle can start a problem that only gets noticed later in the workflow.
Lost potency doesn’t just mean lower yields. In fields like microelectronics or specialty polymers, contaminated output can cause product recalls, damaged equipment, or worse. It can take just a single off-spec drum to send weeks of production into the trash.
Experience shows that safe storage isn’t complicated, but skipping steps can turn costly really fast. A working lab sticks by these basics: buy only as much as you’ll use in a year, refrigerate between 2°C and 8°C, seal bottles right after use, and keep containers dry. Every time someone in the lab doesn’t cut that corner, the team avoids ruined experiments, failed QC checks, or equipment cleanup.
In an industry where quality standards rise every year, these habits shield both people and projects. Mistakes with silanes rarely stay small, so a little extra care up front saves money, time, and reputation in the long run.
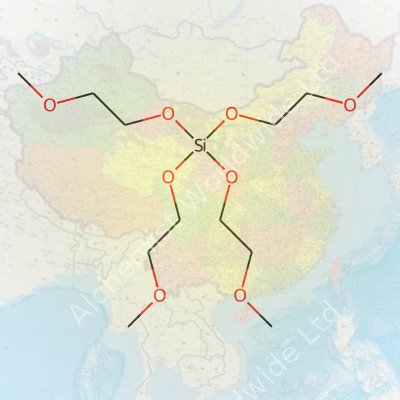
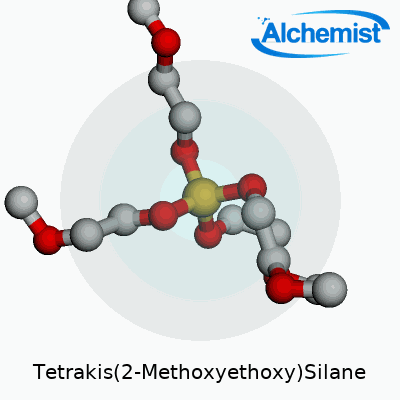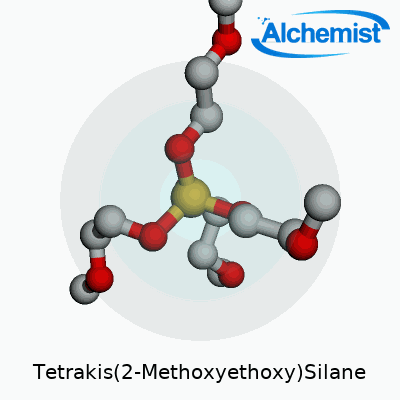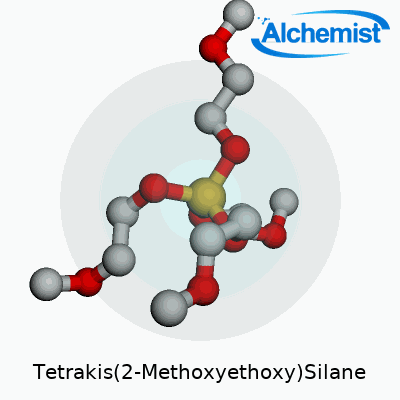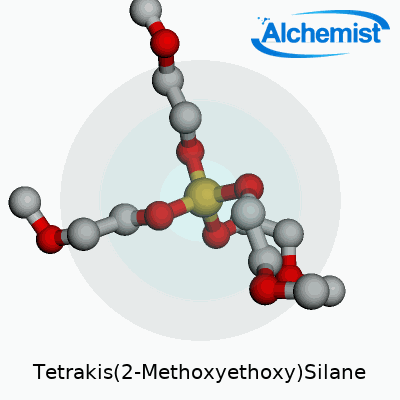
| Names | |
| Preferred IUPAC name | Tetrakis(2-methoxyethoxy)silane |
| Other names |
Tetrakis(2-methoxyethoxy)silane 2-Methoxyethoxy silane Silane, tetrakis(2-methoxyethoxy)- Tetra[2-(2-methoxyethoxy)]silane |
| Pronunciation | /ˈtɛtrəˌkɪs tuː ˌmɛθ.ɒk.siˈɛθ.ɒk.si ˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 18765-38-3 |
| Beilstein Reference | 1698737 |
| ChEBI | CHEBI:87140 |
| ChEMBL | CHEMBL4297054 |
| ChemSpider | 21775118 |
| DrugBank | DB16699 |
| ECHA InfoCard | ECHA InfoCard: 21-2119981507-34-0000 |
| EC Number | 213-207-7 |
| Gmelin Reference | 1276874 |
| KEGG | C18606 |
| MeSH | D017209 |
| PubChem CID | 24866184 |
| RTECS number | VV5950000 |
| UNII | GV8Y7B0B2V |
| UN number | UN2660 |
| Properties | |
| Chemical formula | C12H28O8Si |
| Molar mass | 376.6 g/mol |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 1.08 g/mL at 25 °C |
| Solubility in water | soluble |
| log P | -1.0 |
| Vapor pressure | <1 mmHg (20 °C) |
| Acidity (pKa) | 7.7 |
| Basicity (pKb) | No data |
| Magnetic susceptibility (χ) | -77.0e-6 cm³/mol |
| Refractive index (nD) | 1.409 |
| Viscosity | 15 cP (25 °C) |
| Dipole moment | 2.13 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 504.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1738.65 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS05 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P370+P378, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | Flash point: 110 °C |
| Autoignition temperature | 290 °C |
| Lethal dose or concentration | LD50 Oral Rat 8500 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2,000 mg/kg |
| NIOSH | GB9625000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 3 ppm |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Tetraethoxysilane Tetramethoxysilane Tetra-n-butoxysilane Tetrakis(2-ethylhexoxy)silane Tetrakis(2-hydroxyethoxy)silane |