
The story of Tetramethyl Orthosilicate, often referred to as TMOS, stretches back over a century. Chemists started dabbling with silicon alkoxides in the late 1800s, but it wasn’t until the postwar period that TMOS started landing on the radars of industry and academic labs. Research groups in the 1950s and 60s discovered that TMOS offered unique properties for the production of advanced ceramics, specialty glasses, and sol-gel materials. I remember reading older research papers where TMOS stood out for its reactivity and the purity it could deliver in silicon-based applications. This reputation helped it move from bench-scale curiosity to a key reagent for coatings, semiconductors, and aerogels. The industrial push for high-performance materials during the computer boom kept demand strong, laying the groundwork for the extensive technical datasheets and supply chains we see today.
TMOS shows up in bottles as a clear, colorless liquid. Chemists often describe its sharp, ether-like smell, which reminds me of walking into a freshly cleaned lab. Manufacturers sell it under names like Methyl Orthosilicate, Silicon Tetramethoxide, or even Ortosilicato de Tetrametilo, depending on where you shop. Producers usually list purity levels (often above 98%) and specific gravity to signal quality. TMOS isn’t just one of many silicon alkoxide chemicals; it wins favor for its fast hydrolysis and ability to give high-quality silica networks. Production usually keeps contamination low, so downstream users—whether working on electronics, coatings, or optical fibers—don't spend time on unnecessary purification.
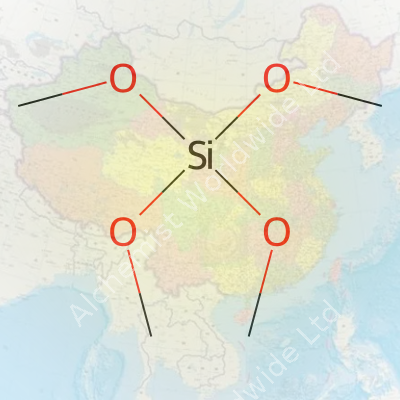
TMOS has a molecular formula of Si(OCH3)4, and its molar mass clocks in at about 152.22 g/mol. This liquid boils at roughly 121 degrees Celsius, so it evaporates under moderate heating. The density hovers around 1.03 g/cm³. If you’ve ever spilled some TMOS in the lab, you know how fast it reacts with moisture in the air—forming a white cloud of silica and methanol. TMOS dissolves easily in organic solvents like ethanol, toluene, and acetone. Some chemists like this rapid reactivity; others have learned the hard way to keep it tightly closed so it doesn’t ruin expensive precursors in shared chemical stores.
Labels on TMOS drums draw attention to flammability, toxicity, and handling hazards. Every container sports clear UN numbers and hazard pictograms due to strict shipping regulations. The material safety data sheet describes storage between 2 and 8 degrees Celsius, away from water and open flames. Specifications will detail residual moisture, maximum levels of metallic impurities, and acceptable methanol content—all crucial for electronics-grade applications. Reputable suppliers often include batch analysis data, which helps avoid surprise impurities that could tank a reaction or mess up a surface-coating process.
Industrial production of TMOS usually starts with silicon tetrachloride and methanol. The reaction releases methyl chloride and forms TMOS as the main product. When I’ve seen the process in action, the importance of dry conditions really stands out. No manufacturer wants their TMOS batch to foam with white silica or go cloudy, so they run the reaction under a nitrogen atmosphere, using glass-lined kettles and careful, staged additions. After the main reaction finishes, distillation under reduced pressure removes side products. In the lab, smaller syntheses skip some steps, but water control still rules every move.
TMOS acts like a hardcore version of tetraethyl orthosilicate (TEOS), thanks to its faster hydrolysis rate. Exposed to water, TMOS goes through swift hydrolysis and condensation, producing dense silica gels and methanol. Researchers have tuned its reactions to create fine-particle silica, open-porous aerogels, and hybrid organic–inorganic films. With some clever ligand exchange, TMOS can produce custom-functionalized silicates, valuable in both chromatography and catalysis. High reactivity sometimes causes problems—unwanted gelling or clogged equipment—so skilled chemists watch their conditions closely.
Over the years, I’ve lost count of how many times TMOS gets relabeled in literature and catalogs. Names like Tetramethyl Silicate, Silicon Tetramethoxide, and Methyl Silicate-4 show up on bottles from Sigma-Aldrich, Alfa Aesar, and other producers. European suppliers sometimes stick to the old IUPAC term, Tetramethyl Orthosilicate. No matter the branding, MSDS sheets reflect its hazards and core chemistry.
TMOS doesn’t leave much room for error in the lab or on the shop floor. It breaks down in moist air, releasing methanol and fine silica particles—raising both acute toxicity and long-term health risk concerns. Proper personal protective equipment includes chemical goggles, nitrile gloves, lab coats, and, if you must open decent volumes outside a glovebox, quality fume extraction. Methanol released by hydrolysis poses its own inhalation and ingestion risks, so occupational exposure limits shape factory protocols and regulatory guidelines. Chemical storage rooms keep TMOS far from oxidizers and sources of ignition. Procedures for handling spills and leaks include containment with absorbent materials and immediate disposal under hazardous material rules. Labs investing in TMOS benefit from regular safety training, clear signage, and emergency washing stations.
TMOS holds its reputation in areas like sol-gel processing, advanced glassmaking, and the manufacture of aerogels and xerogels. Semiconductor firms lean heavily on TMOS for thin-film deposition, insulating layers, and microfabrication. Optical fiber manufacturers prize it for purity and reactivity, crucial for stable, high-transmission performance. Its legacy runs through art restoration, where conservators create stone consolidants and moisture barriers. Scientists mixing specialty coatings and binders find TMOS tough to replace—no other silicon alkoxide delivers comparable processing speed or crosslinking density in so many applications. In environmental cleanup, TMOS-derived materials help build nanoporous membranes and filter supports. Its versatility draws in researchers from energy storage, catalysis, and even biomedical engineering, especially for surface modification of nanoparticles.
Academic and industrial R&D teams often chase better control over TMOS’s hydrolysis and condensation kinetics. Over the last decade, real progress has come from tweaking the order of reagent additions, solvent choice, or introducing catalysts and surfactants. Multinational labs report on new hybrid nanostructures and smart surfaces, using TMOS as a starting point for integrating organic groups or bioactive molecules. Collaborative teams publish on “green” synthesis methods, aiming to cut solvent and methanol waste. Patent filings highlight process tweaks that deliver better pore control or improved thermal stability in downstream silica products. I’ve noticed journals reporting on everything from flexible aerogels for space tech to super-thin coatings for anti-fog or self-cleaning windows—usually with TMOS somewhere in the methods section.
Toxicologists focus on two main risks: direct exposure to TMOS vapor and the methanol produced during hydrolysis. Animal studies show that inhalation of TMOS mist or vapor irritates mucous membranes and can damage lung tissue—especially in higher doses or repeated exposures. Methanol, a known neurotoxin, makes even short-term exposure risky without ventilation. In industry, every training manual I’ve read puts engineered controls front and center, since vapor or droplet formation happens quickly and unpredictably. Environmental studies suggest TMOS breaks down in air and water, but off-gassing methanol raises pollution and health concerns for communities near big production sites. Regulators watch disposal practices closely, so improper dumping ends up as headlines and fines. The demand for safer alternatives and process modifications keeps toxicology research active—especially in labs driving toward greener, methanol-free silicon technologies.
Every year, journals and conference talks highlight new angles for TMOS. As chip fabrication pushes for ever-cleaner, denser interlayers, demand for ultra-high purity TMOS keeps rising. Renewable energy projects turn eyes toward robust TMOS-derived gels for safe, stable battery membranes. Material scientists see opportunity for new silica aerogels in lightweight insulation—cutting building energy costs in cold climates. Cleaner production routes garner attention, using alternative alcohols or reducing formation of hazardous byproducts. I see growing industry collaboration with universities, targeting circular processes for TMOS waste recovery and methanol recycling. As global tech advances, so does the expectation for materials like TMOS not just to perform, but to leave a lighter environmental and health footprint. This push for better outcomes keeps talented chemists, engineers, and regulatory specialists focused on innovation, safety, and long-term sustainability of key building-block chemicals like Tetramethyl Orthosilicate.
Tetramethyl orthosilicate, or TMOS as many in laboratories call it, doesn’t make headlines, but it quietly shapes a lot of what we encounter in technology and production. TMOS belongs to a group of chemicals called organosilicates, and just the smell reminds me of the early days in chemical research—cautious pipetting, a faint hint of solvent, and that keen awareness of safety goggles. But this colorless liquid has real impact. I’ve watched researchers transform it into the clear coatings and nanoparticles powering the next generation of electronics and industrial products.
TMOS has a knack for building up strong, resilient silica networks. Factories and labs use it to form thin, pure, high-quality silicon dioxide layers—essential for everything from protecting microchips to strengthening medical instruments. Anyone who’s handled growing a sol-gel film by hydrolyzing TMOS knows how vital it is to keep water levels in check. If there’s too much, your film cracks; too little, and you never get the solid glass you sought.
Semiconductor plants rely on this compound to deposit smooth, uniform layers that protect delicate chips from heat or static. The reliability of modern phones and computers owes something to TMOS chemistry. In my experience, the right surface finish from a well-controlled TMOS reaction means fewer defects and surprises down the line.
TMOS rarely stands alone. It joins silicon powders to create reinforced glass for fiber optics, laboratory equipment, and even some paints. Artists working with glass have found that TMOS-based coatings let them bond color and texture to the surface, giving old techniques a fresh edge. I’ve met glassblowers who swear by silica coatings made in small batches in the back of their studio to add brilliance and toughness to their designs.
Water purification systems count on TMOS-derived silica to act as the ultrafine sieves trapping contaminants. On a summer spent watching municipal water engineers in action, it struck me just how quietly TMOS helps keep drinking water safe. Its chemistry allows engineers to control pore size with more precision than stuffing sand into a tube ever could. As clean air becomes a more urgent concern, scientists look to TMOS to craft filters for air-purifiers, removing particles smaller than the eye can see.
Like many versatile chemicals, TMOS asks for respect. Inhalation can irritate lungs, and contact can burn the skin. Nobody reaches for TMOS without gloves or airflow hoods. The Environmental Protection Agency recognizes the dangers of careless handling, and workplaces need clear rules about storage, use, and disposal. I’ve found that the best labs and manufacturers constantly retrain workers and double-check procedures. Mistakes can cost health and years of grant-funded research.
As the demand for advanced materials grows, companies and scientists will face pressure to develop safer, cleaner synthesis routes. Preparing silica from rice husk ash or sand offers greener paths, but only research and investment will help scale up alternatives. Consumers can ask more about product sourcing. Engineers and managers who keep safety and sustainability at the forefront keep risks in check while nudging the industry forward. There’s no way around careful stewardship with chemicals as powerful as TMOS.
Tetramethyl orthosilicate, often called TMOS, holds a reputation as a go-to chemical in making glass, coatings, and even certain electronics. I once worked with a team developing nanoparticle coatings, and TMOS played a role in the lab for its ability to produce high-purity silica. Its volatile nature made handling it more nerve-wracking than most materials.
Few realize that TMOS, though helpful in industrial processes, comes with real health risks. Breathing it in—even briefly—can trigger strong irritation in the nose and throat. It can sting the eyes and cause skin to redden. With enough exposure, lungs take the brunt of the damage. Reports show that inhaling TMOS vapors can spark coughing, chest pain, and trouble breathing. The real danger comes when TMOS breaks down in the air or inside the lungs. It releases methanol, a chemical that can damage nerves and organs. Once, during a maintenance shutdown, a colleague overlooked a leaking bottle; the sharp smell, like alcohol but harsher, lingered, and he left with headaches and nausea that hung around for days.
Most stories in chemistry labs don’t get told outside the workplace. The scary part is that TMOS can leave long-term damage from regular, low-level contact. Silica dust, formed after TMOS breaks down, has a notorious reputation for causing lung scarring or silicosis—a condition that cuts down on a person’s ability to breathe over years. Inhaling the stuff, even before it settles as dust, can set off inflammation and tissue damage in the lungs. Data from the CDC and NIOSH tracks links between TMOS exposure and occupational illnesses, supporting concerns raised by workers’ stories. These facts set off alarm bells in refinery settings where faulty exhaust systems and spotty personal protective equipment turn minor leaks into crises.
Simple fixes go a long way. Good ventilation and real-time monitoring tools help catch leaks before they start trouble. Workers who see the value in regular training avoid risky shortcuts and recognize the warning signs. Based on my own drills in the paint and coatings industry, I found a big difference in risk when staff respected handling rules and wore the right masks and gloves. Emergency showers and eyewash stations sound like overkill until one day someone fumbles a container and regrets being unprepared. Regulatory guidelines line up with these practical steps. OSHA and NIOSH recommend airborne TMOS concentrations stay below 0.016 ppm. That’s a tiny amount, but it signals how even small mistakes matter.
Chemical safety requires constant vigilance. Some companies have started switching to alternatives or tweaking processes to cut out TMOS, especially in research or pilot-scale applications. It takes investment and commitment, but it pays off in worker health. Even as Tetramethyl orthosilicate serves industry needs, stories from shop floors and cleanrooms offer real reminders that no job is worth a lifetime health problem. Everyone from supervisors to new hires needs to recognize these risks, demand transparent reporting, and press for better safeguards at every turn.
Tetramethyl Orthosilicate (TMOS) isn’t just another lab chemical you shuffle onto a crowded shelf. Many in the sciences know TMOS for its role in coatings, electronics, and making sol-gel materials. This compound looks harmless enough — a clear, colorless liquid, no wild colors or ominous fumes. But that’s part of why it deserves respect. You won’t get warning signs like a strong smell or visible vapors before things go sideways. It evaporates quietly, and the vapors can catch fire, or cause lung injuries. The colorless look tricks newcomers into underestimating it.
The fire risk grabs attention right away. TMOS has a flash point around 10°C (50°F). That beats plenty of household paint thinners. If you store it where temperatures get cozy, you’re giving flammable vapors a head start. Many recall stories in research labs where ventilation failed or where a bottle cracked in a stuffy stockroom. Those rooms had to get shut down and cleaned up by hazmat crews. Just one mistake stirs up a mess. It’s not just fire, either. Breathing in TMOS vapors can burn your respiratory system. It travels deep into the lungs, making it just as risky for folks moving boxes as for bench chemists.
Safe storage always starts with a cool, dry location away from heat sources. That means no shelves above radiators, no windows that get direct sunlight, no next-door neighbors that give off heat. A spot with a tight temperature range, ideally between 2°C and 8°C, makes a huge difference. Many labs prefer purpose-built flammable liquids cabinets for this job. Those metal cabinets, built with self-closing doors and thick insulation, keep heat and flames out and lock up the vapors. Simple locked closets won’t cut it.
TMOS reacts with moisture — water in the air starts breaking it down. Those glass bottles you see in the back of supply fridges come with airtight seals for a reason. A cracked or loose cap lets in water vapor, turning TMOS to silica gel and methanol. That’s a mix nobody wants to breathe, and it clogs up equipment, too. Old timers in chemistry will tell you: if your TMOS has a white crust around the opening, it’s already been compromised.
The label tells you who made it and when it went on the shelf, but it also needs a hazard warning. A clear, sturdy tag lets your coworkers know what’s inside before they pick up the bottle. No one wants to grab something in a hurry and end up with chemical burns or a lockdown. Training makes accidents less likely. Team members need real hazard drills — not just a printed safety sheet buried somewhere. I’ve seen teams practice spill response using colored water so they’re not fumbling the day it’s real TMOS.
Old, leftover TMOS should never sit around in leaky containers. Every time you walk by old supplies, get rid of what’s expired. A good chemical hygiene plan gets everyone checking storage and using secondary containment — trays that catch drips or leaks. It’s cheaper than cleaning up after a disaster. Make sure a spill kit sits within reach that deals with flammable liquid spills. That should include absorbent pads, proper gloves, and a plan for waste collection. Any slipups threaten health and spark regulatory headaches.
Safe storage habits don’t just keep regulators happy — they protect everyone who sets foot in the workspace. TMOS rewards preparation and punishes shortcuts. That’s a lesson best learned before something goes wrong.
Tetramethyl orthosilicate grabs attention in glass and coatings labs. Its vapors sting, and the stuff irritates the skin. Handling it without the proper respect could land you with burns or a harsh cough that might linger for days. I've seen first-time interns underestimate it, thinking gloves alone offer full protection. Years of working in research taught me: bad habits in the lab catch up fast.
Pouring tetramethyl orthosilicate releases vapors that sting your nose and eyes right away. Good fume hoods aren’t “nice to have”; they’re non-negotiable. I’ve watched experienced colleagues pour even tiny amounts inside a hood. One time, a grad student tried skipping this step to save time, opening a bottle outside the hood. Red eyes and a persistent headache haunted him most of the week.
I’ve tried thick nitrile gloves with this chemical—thin ones break down in no time. Splash goggles seal tight, since vapors can go around glasses and irritate your eyes before you even notice. Some labs push for full face shields, and after seeing a splash eat through a lab coat, I understand the reason. Cotton lab coats, long pants, and closed shoes block splashes that could soak right through synthetic fabrics.
Chemical shelves fill up fast, but tetramethyl orthosilicate doesn’t belong in the middle of the pack. It must stay away from acids, water, and moisture. I once saw a half-closed container swell from inside, popping the lid because someone left a wet rag nearby. This chemical reacts with water, forming tough gels and releasing methanol—a toxin that sneaks up on your nervous system.
Heavy spills cause panic only if you aren’t prepared. Every lab using this chemical should keep absorbent pads and sand ready to trap the liquid right away. In my years handling spills, rushing to clean with the wrong material only spread the mess or made the vapors worse. Never use water—dry absorbents only. Once contained, waste heads for hazardous disposal. Keeping your emergency phone numbers posted within reach makes sense here.
Open flames and even hot plates make the situation dangerous. Methanol vapor forms when tetramethyl orthosilicate meets water, and even a spark can trigger a fire. I keep my workspace clean, with no paper towels or solvents lying around, to limit fuel for any accidental ignition.
Mastering safe handling starts with training. I remember fumbling my first year, double-checking each bottle and sweating through safety drills. Over time, it becomes a flow: label-check, gloves on, hood open, measuring done, tools wiped. Sharing stories and lessons with coworkers builds that safety culture. Each reminder saves stress the next time someone grabs the bottle in a hurry.
Safety matters most before trouble starts. Choosing the right storage, keeping cleanups close, and training the team all cost less than treating injuries or replacing ruined samples. Respect for these basics keeps everyone working—healthy, focused, and with all ten fingers right where they should be.
Tetramethyl orthosilicate, known by its chemical formula Si(OCH3)4, turns up in lots of labs and factories. I’ve crossed paths with it during research projects and plant visits. It comes across as a clear, colorless liquid, and the stuff draws a crowd because of its versatility. Anyone working with glass coatings, silicone polymers, or specialty ceramics recognizes its value. In my years talking with chemists and plant managers, I noticed safety goggles came out fast once someone mentioned this compound. That’s partly because of its reactivity—water exposure jumps it straight into forming silicon dioxide and methanol.
I once visited a research team at a university, where a young scientist was frustrated over contamination problems in sol-gel synthesis. His group turned to tetramethyl orthosilicate for its high purity and reliable behavior during hydrolysis. Si(OCH3)4 helped them lay down ultra-thin films for microelectronic devices. From manufacturers I know, it’s almost standard for producing artificial opals, protective glass coatings, and even catalysts for chemical reactions. The key comes from the tetrahedral silicon atom at the center, bound to four methoxy groups, which lets it deliver silicon atoms with impressive precision.
Having worked in places using TMOS (as regulars call it), I appreciate the need for real caution. Breathing its vapors or even a skin splash can spell trouble—causing burns, eye irritation, and toxic methanol byproducts once it hydrolyzes. I learned the hard way during an internship: even a few drops on the bench gave off fumes strong enough to make my eyes water. Real-world practice means never skipping gloves or fume hoods, and I’ve seen whole labs adopt stricter procedures after minor accidents. These stories underline the need for solid protocols and staff training, especially since mistakes can turn serious very quickly.
Tetramethyl orthosilicate doesn’t just vanish once it leaves the container. I’ve spoken with waste management experts who stress that the methanol formed after hydrolysis can pollute water supplies, and the leftover silica might not decompose cleanly in every landfill. Facilities must capture and neutralize both TMOS and its byproducts, meeting local disposal rules. Real progress depends on both regulators and industry leaders finding common ground. Between accidental spills and growing demand, many believe real investment in better capture technologies is overdue.
The path forward draws on practical changes. Training for all staff, not just chemists, needs more time and focus. My best safety training moments came from hands-on sessions, not just dry manuals. Facilities could cut accidents in half with clear signage, real-time sensors for methanol leaks, and sturdy gloves. Some companies pivot toward greener alternatives, but others press for stricter airtight handling systems and better recycling programs. The call for innovation is growing louder, and prizes often go to the first team reducing emissions or reusing byproducts.
Si(OCH3)4 offers undeniable benefits to industry and science, but its hazards and environmental impact mean every user should handle it with real care. Investment in safety, waste solutions, and hands-on training leads to safer jobs and cleaner outcomes. Sharing lessons learned—both successes and slip-ups—pushes the community toward safer, cleaner practices.
| Names | |
| Preferred IUPAC name | tetramethyl silicate |
| Other names |
TMOS Tetramethyl silicate Tetramethoxysilane Orthosilicic acid tetramethyl ester Methyl silicate |
| Pronunciation | /ˌtɛtrəˈmɛθɪl ˌɔːrθə.sɪˈlɪ.keɪt/ |
| Identifiers | |
| CAS Number | 78-10-4 |
| Beilstein Reference | 2081233 |
| ChEBI | CHEBI:132870 |
| ChEMBL | CHEMBL158967 |
| ChemSpider | 7759 |
| DrugBank | DB11200 |
| ECHA InfoCard | 100.003.239 |
| EC Number | '208-940-9' |
| Gmelin Reference | 85240 |
| KEGG | C06542 |
| MeSH | D013742 |
| PubChem CID | 6626 |
| RTECS number | VV7325000 |
| UNII | J50ISB4OZR |
| UN number | UN1992 |
| Properties | |
| Chemical formula | C5H12O4Si |
| Molar mass | 152.23 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Pungent |
| Density | 0.96 g/cm3 |
| Solubility in water | Moderately soluble |
| log P | 0.2 |
| Vapor pressure | 1.2 hPa (20 °C) |
| Acidity (pKa) | 2.8 |
| Basicity (pKb) | pKb: 7.18 |
| Magnetic susceptibility (χ) | -59.0e-6 cm³/mol |
| Refractive index (nD) | 1.382 |
| Viscosity | 0.54 mPa·s (20 °C) |
| Dipole moment | 3.27 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 276.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1363 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3338.8 kJ/mol |
| Pharmacology | |
| ATC code | V09CX04 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H226, H301, H311, H331, H319, H335 |
| Precautionary statements | H260, H301, H311, H331, H314, P210, P222, P231+P232, P260, P264, P270, P271, P280, P301+P310, P303+P361+P353, P304+P340, P305+P351+P338, P311, P320, P330, P363, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-2-W |
| Flash point | 21 °C |
| Autoignition temperature | 430°C |
| Explosive limits | 1.3% - 22.8% (in air) |
| Lethal dose or concentration | LD50 Oral Rat 6270 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 8500 mg/kg |
| NIOSH | SI8580000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Tetramethyl Orthosilicate: "1 ppm (5 mg/m3) (OSHA PEL) |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Tetraethyl orthosilicate Tetrapropyl orthosilicate Tetrabutyl orthosilicate Silicon dioxide Trimethoxysilane |