
Tetrapropoxysilane came into the research spotlight during the growth of organosilicon chemistry in the twentieth century. Once scientists realized the promise of silicon-based chemicals for industrial coatings, adhesives, and electronics, the race to discover new silane derivatives kicked off. Early work honed in on tetraalkoxysilanes, with tetrapropoxysilane drawing attention for its longer, branched propoxy chains compared to its methoxy or ethoxy cousins. Laboratories across Europe and the United States scoured for methods to reliably synthesize these silicon esters, tracking yields, costs, and stability. Practicality proved as important as novelty. As the chemical industry grew in the 1960s and 1970s, large-scale processes for producing silanes, including tetrapropoxysilane, became commercial realities, changing the landscape of specialty materials.
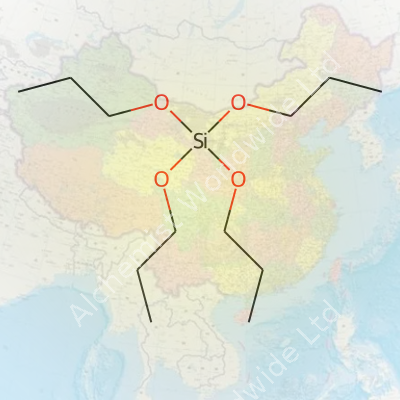
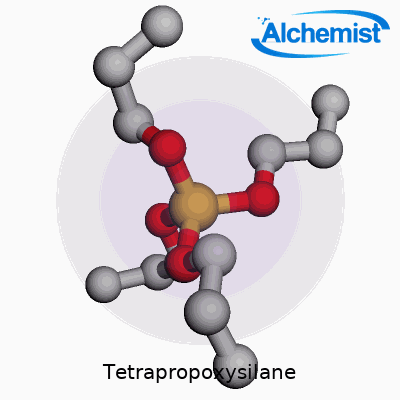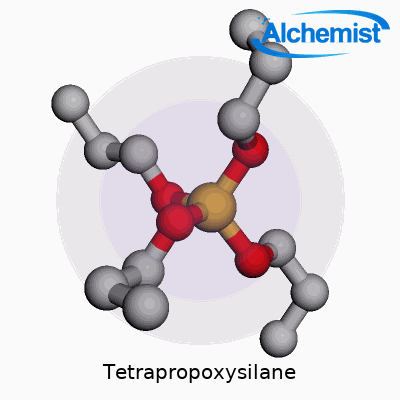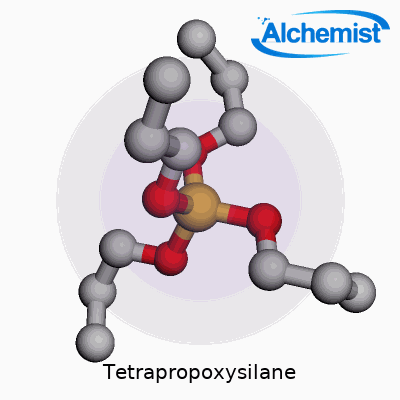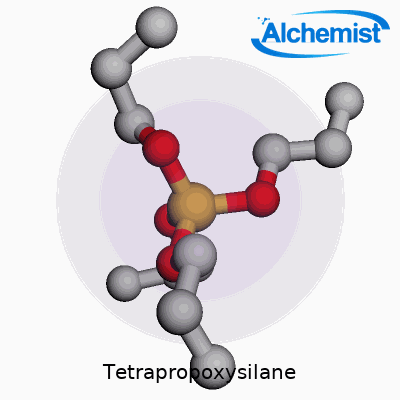
In simple terms, tetrapropoxysilane is a silane compound, structured as Si(OC3H7)4. This means a central silicon atom connects with four propoxy groups. These longer organic arms bring the substance a number of distinctive qualities. Unlike shorter-chained analogs, tetrapropoxysilane brings a different set of handling and formulation dynamics, which underpins its value for researchers and manufacturers hunting for specific performance attributes in coatings, sol-gel films, and SiO2-based materials. Its role often involves acting as a source of silicon dioxide or a binding agent, especially in advanced materials that demand precisely tailored porosity, adhesion, or hydrophobicity.
Tetrapropoxysilane appears as a colorless, faintly aromatic liquid, with a boiling point hovering around 235–238°C at atmospheric pressure. Compared to its methyl or ethyl counterparts, its higher molecular weight and longer alkoxy chains create lower volatility and a greater viscosity. It does not mix with water, owing to hydrolysis reactions that rapidly break it down, but it blends well with many common organic solvents like toluene or alcohols. Chemically, the silicon–oxygen bonds in each propoxy group shape much of its reactivity. The compound tends to stay stable in dry or non-acidic environments but reacts quickly with moisture, leading to the gradual building of silica networks—a core reason for its popularity in sol-gel processes and films.
Industry producers supply tetrapropoxysilane in tightly sealed, often nitrogen-flushed drums to prevent unnecessary contact with air moisture. Purity commonly falls in the 98–99% range, with water content usually held below 0.2%, and acid values kept as low as practical to avoid premature breakdown. Common labeling callouts include chemical identity “Tetrapropoxysilane,” UN number (if transport classifications apply), synthesis batch, net weight, and hazard pictograms for flammability and health. Some regulatory jurisdictions request detailed hazard communication sheets, spelling out risks, first-aid protocol, and storage guidelines, reflecting a shift in global practice toward greater transparency for industrial chemicals.
Manufacturers usually prepare tetrapropoxysilane by reacting silicon tetrachloride with propanol under controlled conditions. The basic approach: silicon tetrachloride drips gradually into excess propanol while maintaining cooling and rapid stirring. As the reaction progresses, hydrogen chloride gas bubbles off and can pose a corrosion or inhalation hazard. Several hours’ worth of purification—typically distillation under reduced pressure—cleans up the crude silane. Each step in the process requires attention to moisture control. I’ve seen how a stray drip of water can turn a promising batch into sticky, unusable silica, wasting time and resources. Technicians monitor temperatures, check the purity of reactants, and often run tests measuring residual chlorine, which, if left unchecked, may damage downstream applications.
One of the key attributes of tetrapropoxysilane comes from its reactivity with water. Expose the compound to a touch of humidity, and it starts a cascade—hydrolysis yields silanol groups, which then condense to form intricate silica networks. This reaction forms the backbone of sol-gel technology, allowing the design of films, powders, and coatings with precise porosity and tailored mechanical strength. The longer propoxy chains slow this hydrolysis compared to methyl or ethyl analogs, enabling a more controlled build-up of the final structure. Chemists sometimes tweak the base silane, swapping out propoxy for other groups to tune solubility or reactivity. Crosslinking modifications open up more specialized uses, such as hydrophobic coatings or organosilicon resins for high-performance polymers.
In industry and literature, tetrapropoxysilane often turns up under a handful of synonyms: Tetrapropyl orthosilicate, silicon tetrapropoxide, or its systematic label, tetrapropoxy orthosilicate. Suppliers sometimes bundle it into product codes or proprietary trade names, but the base compound stays the same. This variety in naming can create confusion, especially for new chemists or procurement teams. Diligence with CAS numbers during sourcing avoids supply missteps. This has always been crucial for me, since even a minor mix-up in silicon source can wreck a formulation or force expensive troubleshooting.
Handling tetrapropoxysilane asks for respect. Its vapors irritate eyes and skin, and contact with water brings not just chemical burns but risk of glass-like silica dust developing right on site. Good labs run well-ventilated hoods and keep multi-layer gloves and goggles as the norm, not an afterthought. Transport standards, especially for bulk quantities, lean on rigorous containment and hazard communication. Some companies bundle real-world simulations into their safety drills, walking staff through what happens if a drum leaks or a splash lands on unprotected skin. Over time, I’ve seen safety culture make the difference between a minor scare and a serious incident, highlighting the payback of clear protocols and steady, thorough training.
Tetrapropoxysilane now powers a whole spectrum of industries. In microelectronics, thin uniform silica layers protect circuits from heat and humidity. In coatings, its unique hydrolysis profile lets chemists build films that ward off corrosion or boost scratch resistance. Additive manufacturers often use it for glass fiber or foam-based insulation. Some specialty artists and restorers even chase after it for stone conservation, banking on silica networks to bond loose grains in historical artifacts. Years in R&D taught me the biggest breakthroughs often spring out of cross-pollination: pulling a silane from one sector’s toolkit and reimagining its use elsewhere, often with only subtle tweaks in the process or the mix of ingredients.
R&D teams have put tetrapropoxysilane under the spotlight as a tool for fine-tuning nanostructured materials, sensors, and functionalized surfaces. Today, bottom-up engineers lean on its slower hydrolysis to give them a gentler reaction that preserves sensitive substrates or allows in-situ doping. Researchers dig deep into parameters like pH, catalyst, and solvent mix, drawing up reams of data on particle size distribution and network growth. The compound has helped open up black boxes in sol-gel chemistry, with scientists using advanced microscopy and spectroscopy to watch the very first moments of silica cluster growth. This curiosity-driven work keeps spinning new ways to deploy silane chemistry far beyond its original industrial boundaries.
Toxicology studies reveal that tetrapropoxysilane can cause real harm with direct or prolonged exposure. Inhalation of vapor or accidental spills causes immediate discomfort, while chronic exposure brings risks of organ damage, especially in poorly regulated settings. Data collected over decades feeds into regulatory frameworks worldwide—OSHA, REACH, and others set exposure limits not just as a bureaucratic step, but out of recognition for how potent these organosilanes can be. Routine health monitoring, environmental fate studies, and updated material safety data sheets all keep end users and nearby communities safer. Honestly, even as new data comes out, the focus on reducing risk, rather than just meeting paperwork standards, strikes me as a lasting lesson from the industry’s more troubled chapters.
Forward-looking companies see tetrapropoxysilane sitting at the edge of some big shifts in material science. Interest is building around greener production, with efforts on renewable raw materials and closed-loop solvent recovery both driven by regulations and real concerns over waste. Functional coatings for renewable energy, self-cleaning surfaces, and additive manufacturing could drive demand for highly controllable silane chemistry. Quantum dot technology, flexible displays, and nanoelectronics each demand new grades of purity and reactivity—areas where tweaks to old silane favorites pay dividends. I get the sense that, even after decades in the game, this compound’s best days could still be ahead, provided scientists and industry stay open to fresh ideas and remain vigilant on the health, safety, and environmental front.
Tetrapropoxysilane sounds like something you’d only find in a lab, maybe somewhere behind locked doors and warning signs. In truth, it shows up in products and technologies that shape everyday life. One of its core roles comes from how it helps build things from the microscopic level up—especially in electronics, coatings, and advanced materials.
If you’ve ever pulled apart a broken smartphone, you’ll notice delicate chips, wafers, and boards packed tighter than a downtown parking lot. Creating those microstructures relies on certain chemicals, and tetrapropoxysilane belongs in that toolbox. Manufacturers use it during the process called “chemical vapor deposition,” where it lays down a thin layer of silicon dioxide. This isn’t just technical jargon—the layer acts as insulation and a gatekeeper, keeping signals clear and components steady. Without reliable insulators, today's computing power would hiccup and crash. Demand for chips soared during the pandemic, pulling attention to how crucial materials like this are.
Glass manufacturers use tetrapropoxysilane in what's called the sol-gel process. Instead of shaping objects only by melting sand at outrageous temperatures, companies can use chemicals like this one to assemble glass or ceramic layers molecule by molecule. That leads to precise coatings for everything from camera lenses to touchscreens. There’s a personal angle here, too—better coatings mean fewer scratches on glasses and gadgets, grounding the importance of this chemical in the stuff we deal with every day. In construction, treated glass lasts longer against weather and grime, holding up against storms instead of needing constant replacement.
As a dad and someone who tinkers in the garage, I always read materials safety sheets before working with new chemicals. Tetrapropoxysilane releases dangerous vapors if handled the wrong way. In the workplace, keeping good ventilation and proper training protects workers from breathing in fumes, and serious chemical burns can happen without respect for the risks. Europe and the US require special handling and disposal rules for tetrapropoxysilane, tracking imports and limiting exposure. This helps keep communities safe and builds public trust in high-tech manufacturing.
Pushing for more sustainable production isn’t just a trend. Companies making and using tetrapropoxysilane already look at ways to recover byproducts so waste doesn’t pile up. Some research groups work on alternative precursors that break down faster or use less energy. Investing in closed-loop systems for chemical recycling can further cut releases into the environment. Governments offer incentives for green chemistry approaches; adopting them pays off on the balance sheet and the local ecosystem.
Every year, demand for electronics and durable, high-performance materials grows. Tetrapropoxysilane plays a quiet but consistent part in that growth. The need for cleaner, safer, and more reliable manufacturing pushes both regulation and innovation. Real progress comes from curious minds: researchers, engineers, and frontline workers who spot weak points and chase smarter solutions. As more people look under the hood of modern tech, they’ll find compounds like this matter a lot more than the language on a label might suggest.
Tetrapropoxysilane isn’t just another specialty chemical stashed in a backroom. This compound, used in semiconductor and material science labs, brings its own brand of risks to any workspace. Damp air spells trouble. Exposure to moisture triggers rapid hydrolysis, which not only wastes the chemical but also releases alcohol vapors—breathing those indoors feels like walking into a strong solvent cloud. Safety data consistently flag respiratory irritation, so anyone who’s worked around solvents understands why direct contact is out of the question.
Gloves and goggles might feel cumbersome, but they beat the alternatives. I always reach for nitrile gloves before opening even a sealed jug of Tetrapropoxysilane. Shortcuts with basic protection lead to chemical burns or eye injuries. Even after years working in research labs, I keep fresh eye wash on hand because a split second without protection can mean an expensive trip to urgent care. Fume hoods shouldn’t gather dust; they’re the one spot in a lab meant for handling volatile silicon compounds like this one.
Cool, dry, and out of bright light makes a big difference. You’ll never find Tetrapropoxysilane stored under a sink or next to a radiator in facilities that get audited. Fluctuating temperatures break it down faster than most people guess, and cardboard boxes just don’t cut it as protection. Sealed glass or approved metal containers with gasketed lids keep out air and moisture, which slows any unwanted reactions. I’ve seen leaky stoppers turn a perfectly fine product into a sticky mess in less than a week.
Chemical compatibility matters. I keep flammables, oxidizers, and acids separate—no point risking extra hazards from accidental mixing. Separating incompatible materials isn’t an abstract instruction from a dusty manual; it’s based on real spill incidents that force entire rooms to shut down for remediation. Once I left a bottle near an acid shelf, and vapors led to corrosion on the cap. That sort of mistake only needs to happen once before it sticks with you.
Everyone in the lab or warehouse deserves more than a one-minute briefing. Comprehensive training, clear signage, and up-to-date Safety Data Sheets (SDS) should never feel like overkill. OSHA and similar bodies get strict because so many injuries come from skipping small steps. I’ve walked into places where folks labeled everything with sticky notes. That lasted until someone mixed up containers and ruined thousands of dollars in inventory. Digital tracking systems and well-labelled shelves help keep things in order, and they save time when audits roll around.
Most fires from organosilicon compounds start with simple oversights: one spark, one spill, or one unlabeled shelf. Correct spill kits, absorbent pads, and fire extinguishers tailored for flammable substances keep small mistakes from turning into headlines. Regular checks, plus rotating old stock out, prevent surprises. Tetrapropoxysilane won’t wait for a convenient moment to react; it acts fast in the wrong conditions, and people working with it need to do the same.
I’ve worked on projects where chemical names like tetrapropoxysilane buzz around the lab. The name sounds intimidating, but it’s a silicon-based compound. Chemists use it mostly for coatings, adhesives, and sometimes in electronics. On paper, it helps products gain strength or last longer. The real question always pops up—what price does that extra durability come with?
I’ve seen safety data sheets for tetrapropoxysilane, and safety teams don’t treat it lightly. Direct skin contact burns. Inhalation brings irritation—coughing, sore throat, and lung trouble in high doses. If it splashes in eyes, fast action prevents lasting damage. This isn’t a compound meant for anyone without protective gear. Most work sites call for lab coats, gloves, splash goggles, and enough ventilation to keep the fumes away from lungs. Anyone skipping those steps quickly regrets it. The clear message: workers shouldn’t take shortcuts. People with asthma, skin problems, or eye conditions face more risk. It’s not easy to ignore the basic rule—protect yourself, or pay the price.
In the field, some production lines swap hazardous compounds for safer ones, but tetrapropoxysilane still pops up. The tradeoff always centers on convenience and cost. Most companies put out training videos or live safety demos, but incidents keep happening. Spills bring problems beyond just personal discomfort—hospital visits happen, especially in places where proper protection gets ignored.
From my experience around waste mitigation teams, tetrapropoxysilane rarely makes headlines for major spills, but it doesn’t vanish without consequences. In water, this compound breaks down, but the byproducts can still be rough on organisms—fish, bugs, and plants show stress if exposed. It isn’t friendly to groundwater; over time, leaching makes water treatment harder. In some countries, strict regulations ask for capture systems and special disposal, but enforcement varies a lot.
I’ve watched cleanup teams deploy absorbents and block drains to keep it from spreading. Local wildlife protections often mean more red tape, but not every area has strong oversight. Where oversight slips, so does environmental safety. Regulatory agencies occasionally update their stance, but industries tend to lag behind until fines stack up or contamination makes the evening news.
The search for safer substitutes doesn’t stop. Green chemistry teams look for less toxic options, but the switch isn’t simple. Cost, performance, and existing infrastructure anchor companies to familiar choices like tetrapropoxysilane. Research points to some biopolymers and alternative silicon compounds that promise less risk, but large manufacturing jumps in slowly out of caution or budget fears.
Education seems key. Workers and managers benefit from regular safety refreshers, not just dusty posters on breakroom walls. Shifting attitudes about short-term convenience and long-term well-being could move industries further—especially as new technology widens the pool of safer ingredients.
It takes commitment from leadership, frontline workers, and regulators to keep health and the environment above profit margins. Community input, updated safety standards, and support for research help tilt the table. Sustainable solutions rely on more than checklists or protocols—it’s about changing habits, funding innovation, and pushing for actual transparency about risks. I’ve seen improvement where companies open up, share incident reports, and build a genuine safety culture—the kind that doesn’t wait for a crisis before acting.
Tetrapropoxysilane brings together a silicon atom and four propoxy groups. The chemical formula reads as Si(OC3H7)4. At the core, silicon links up with four oxygen atoms, each tethered to a three-carbon propyl chain. Picture a central silicon atom, each of its four “arms” reaching out to an oxygen, and from each oxygen, a propyl group extends outwards. This layout shapes the molecular geometry and affects how this compound behaves in real-life situations.
Silicon’s role in materials science is hard to ignore. Substances like tetrapropoxysilane can help tailor coatings, insulation, and glass surfaces, especially in demanding work like microelectronics. With this specific structure, propoxy groups shield the silicon core, offering some hydrolytic stability. Exposing this chemical to water or humidity sets off hydrolysis—acid or base conditions speed things up, leading to silanols and propanol, both of which change how a film or coating forms.
Over the years, I’ve seen engineers pick tetrapropoxysilane because the propoxy side chains offer a slower hydrolysis rate than smaller alkoxysilanes such as tetraethoxysilane. This time lag matters for anyone spinning or spraying films on glass or semiconductors. People working with sensitive substrates often need that extra moment to line things up before setting the reaction in motion. These propyl chains help them by slowing things down, letting them fine-tune their work and avoid waste.
In practice, tetrapropoxysilane shows up in sol-gel chemistry. Sol-gel processes change liquid precursors into solid networks, often under mild conditions. This lets manufacturers make thin glass coatings, ceramic fibers, or nanostructures without needing a furnace. Each time propoxy groups break away—especially in an environment where water or alcohols hover—the resulting silica gel changes its final properties. That’s why details like chain length and hydrolysis speed end up so important.
People in research labs and factories watch silica sources carefully, tuning each precursor to achieve an end product with the correct flexibility, adherence, or porosity. Extra time from longer side chains means fewer flaws—valuable when producing electronics where surfaces can’t afford tiny defects. Small tweaks in the structure and formula ripple out, affecting the reliability and lifespan of countless modern devices.
Handling tetrapropoxysilane demands respect for its reactivity. The compound releases propanol during hydrolysis, which brings in workplace safety issues. Proper ventilation and personal protective equipment matter just as much as knowing the chemical formula. Open windows, fitted masks, and gloves become everyday tools alongside precision dispensers and reaction timers.
Some researchers look for greener, safer alternatives. They’re swapping out solvents, changing the reaction environment, or even developing new silicon sources that curb volatile emissions. It’s possible to combine practice with progress by investing in safety training, better storage, and clear labeling. This helps cut down incidents and keeps projects moving without massive interruptions.
Understanding how tetrapropoxysilane’s structure shapes its behavior gives anyone—scientist, technician, or manufacturer—a clear edge. Exact knowledge about the chemical structure leads to safer labs and factories, sharper results, and more sustainable practices. As more industries move to high-performance materials, awareness like this builds a solid foundation for progress and safety.
Tetrapropoxysilane doesn’t pop up in everyday conversations, but its risks can’t be ignored. This silicon-based chemical plays a role in the production of coatings, adhesives, and surface treatments, turning up mostly in industrial settings. Many people don’t realize how dangerous it gets when handled poorly. I’ve experienced firsthand that one oversight around reactive chemicals can spiral into hours of emergency response work. Mishandling leads to toxic vapors, irritating fumes, and hazardous residues, all tough on workers and the local community.
People who think they can flush or landfill Tetrapropoxysilane mix up household and industrial waste. Water reacts aggressively with it, creating clouds of alcohol and corrosive byproducts. Recycling facilities or municipal systems have no way to handle that. More than once, I’ve listened to seasoned disposal experts talk about fires or toxic exposures from amateur dumping. Health data points out respiratory and skin hazards, so local water treatment plants can’t just dilute the material and hope for the best.
Professionals turn to licensed hazardous-waste handlers to get rid of Tetrapropoxysilane. They know two things work: high-temperature incineration and chemical neutralization. High heat cracks the molecule, breaking it down into non-toxic components. Incineration doesn’t mean tossing it in a backyard barrel—industrial-scale combustion takes place under strict temperature and emission controls. Waste facilities that run these systems keep logs, sample their emissions, and stay on the radar of state and federal inspectors.
Some sites manage small volumes with chemical neutralization. These folks add Tetrapropoxysilane slowly to a vessel filled with controlled amounts of alcohol and water in a fume hood. The process gives off heat and potentially flammable vapors, so experts wear respirators, gloves, and eye protection while they mix and vent the reaction. They collect the silanol and propanol byproducts for secondary treatment, often sending them off as hazardous liquids for further incineration.
I know from personal experience that it’s not just about following paperwork or big regulations. Communication and training work best. New employees often ignore tiny spills or splash marks, thinking a quick wipe won’t hurt. Supervisors who train teams to treat every drop as risky see fewer incidents. Labels, warning signs, and sealed drums don’t eliminate danger by themselves. Regular drills and refresher courses shape attitudes long after the training is over.
Demand for stronger waste regulations grows every year, with local governments tightening up their lists of prohibited landfill chemicals. Companies looking to cut costs sometimes search out less-regulated contractors, but that approach rarely works out for long. I’ve watched legal fees and cleanup bills turn a cheap job into a major liability.
The most sustainable fix comes from higher up the production chain. Substitution and process changes keep toxic silanes out of waste containers in the first place. Some manufacturers now test less hazardous precursors or switch to closed-loop systems. Sharing lessons and partnering with emergency responders makes a difference, so everyone knows how to stay safe if disposal mistakes happen.
Practical disposal starts with good science and ends with responsible action. Shortcutting safety brings bigger risks than most people imagine. Safe disposal means less harm, smoother operations, and a real step toward a cleaner environment.
| Names | |
| Preferred IUPAC name | tetrapropoxy(oxosilane) |
| Other names |
Tetrapropoxy silicate Tetrapropoxysilicate Tetrapropoxyorthosilicate Silicic acid tetrapropyl ester Tetrapropyl orthosilicate |
| Pronunciation | /ˌtɛtrə.prəˈpɒk.sɪˌleɪn/ |
| Identifiers | |
| CAS Number | 682-01-9 |
| Beilstein Reference | 1461287 |
| ChEBI | CHEBI:33398 |
| ChEMBL | CHEMBL185159 |
| ChemSpider | 60014 |
| DrugBank | DB11262 |
| ECHA InfoCard | 17c5826d-4b10-441a-9e8a-57bca399a27b |
| EC Number | 213-934-0 |
| Gmelin Reference | 88287 |
| KEGG | C18600 |
| MeSH | C0187108 |
| PubChem CID | 22589 |
| RTECS number | VV5425000 |
| UNII | NMT84L0107 |
| UN number | UN1325 |
| CompTox Dashboard (EPA) | DTXSID4022578 |
| Properties | |
| Chemical formula | C12H28O4Si |
| Molar mass | 332.54 g/mol |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 0.97 g/cm³ |
| Solubility in water | insoluble |
| log P | 2.7 |
| Vapor pressure | 0.3 hPa (20 °C) |
| Acidity (pKa) | 12.5 |
| Magnetic susceptibility (χ) | -67.0e-6 cm^3/mol |
| Refractive index (nD) | 1.407 |
| Viscosity | 1.2 mPa·s (at 25 °C) |
| Dipole moment | 4.04 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 472.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1276.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5234.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | Precautionary statements: "P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-2-2-~W |
| Flash point | 71 °C |
| Autoignition temperature | 460 °C |
| Lethal dose or concentration | LD50 Oral Rat 4000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 4,299 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 200 mg/m3 |
| IDLH (Immediate danger) | IDLH: 250 ppm |
| Related compounds | |
| Related compounds |
Tetraethoxysilane Tetramethoxysilane Tetra-n-butoxysilane Tetraisopropoxysilane Tetrakis(2-ethylhexoxy)silane |