
Silicon-based compounds have followed modern industry from early glass manufacture to the tailored synthetics powering electronics and advanced coatings. Tetrapropylorthosilicate, also called TPOrS, stepped onto the scene as manufacturers looked for ways to control silica formation and minimize byproducts. Early chemists chased improved purity, grinding through solvents and purifying with distillation until they nailed down scalable routes. Over the decades, benchmark processes took cues from earlier work with similar silicon alkoxides, yet TPOrS offered just enough tweak in its molecular makeup to spawn new patents and shift technical roadmaps, especially as specialty glass and catalyst research picked up steam in the late twentieth century. The transition from laboratory batches to bulk industry shipments demanded new thinking in safe transport and rigorous purity standards—another nod to the role of practical problem-solving in chemical innovation.
TPOrS belongs to the family of silicon alkoxides. Chemists look to it as a key precursor in the manufacture of high-performance materials and for modifying surfaces at a microscopic scale. It typically arrives as a clear, slightly viscous liquid. Suppliers package it in drums or steel containers, often under nitrogen to protect against air and moisture. Researchers and engineers consider TPOrS an important stepping stone in catalyst design, sol-gel synthesis, and certain polymer modifications. The product stands out for offering precise stoichiometry in multi-component syntheses, which becomes critical in applications demanding tight control over end properties—be it in coatings, specialty glass, or the ever-hungry electronics sector.
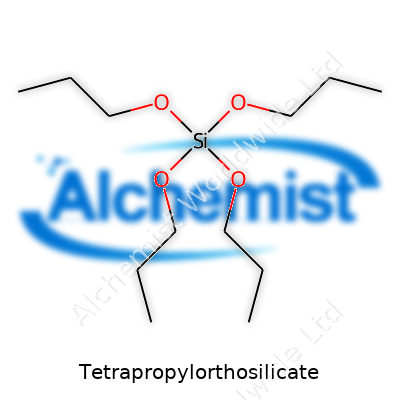
Tetrapropylorthosilicate brings a molecular weight of about 248.43 g/mol. Its formula, Si(OC3H7)4, tells us it packs four propoxy groups bonded to a central silicon atom. The clear liquid carries a faint alcohol scent, which speaks to its propoxy groups and volatility. Its boiling point hovers around 220°C at standard pressure, so short-term heating doesn’t usually trigger rapid vaporization, but careful control remains essential in both storage and application. TPOrS prefers dry conditions since ambient moisture will hydrolyze it, setting off a cascade of chemical changes. Its density sits just shy of water, but the viscosity feels a touch heavier, making transfer slower in large operations. The difference in polarity compared to shorter-chain silicates gives it unique compatibility with organic phases in coating and synthesis processes.
Manufacturers publish clear specifications to help buyers judge fitness for use. Common benchmarks include minimum assay (typically above 98%), moisture content (kept below 0.1% for precision work), color index, and acid number. Impurity levels remain under tight control—alkali metals, chlorides, and iron all sit in the trace range, as even minuscule contamination skews downstream processes. Labels adhere to the Globally Harmonized System, listing hazard warnings, emergency measures, and batch identification. Supplied with certificates of analysis, each shipment guarantees traceability from synthesis to end application. Quality assurance personnel often scan the documentation right at delivery, since one weak link can derail months of process work in high-value industries.
Standard production of TPOrS follows the direct alcoholysis of silicon tetrachloride with n-propanol under anhydrous conditions. Producers add silicon tetrachloride to propanol in a controlled addition, often under cooling, since the reaction gives off heat. Hydrochloric acid evolves during the conversion, so direct sweep with dry nitrogen or vacuum pulls the acid vapor away. To reach high purity, distillation steps remove byproducts, unreacted starting material, and water. Purity ratings above 98% demand scrupulous process control, and technicians routinely sample at each step to track quality. The drive for ever more efficient, greener methods has spurred trials involving catalytic pathways, improved solvent recovery practices, and alternative silicon sources, but the direct alcoholysis route still dominates commercial-scale supply.
Once in the hands of chemists, TPOrS displays classic reactivity for an orthosilicate. Exposure to water or moisture kicks off hydrolysis, splitting the propoxy groups and forming silanols. The resulting intermediates undergo polycondensation, ultimately generating silica networks. This chain of events forms the core of sol-gel processing—building engineered glass, ceramics, and catalyst supports with tightly tuned pore structures. Beyond simple hydrolysis, researchers use TPOrS as a handle for introducing dopants, co-forming hybrid organic-inorganic composites, and even functionalizing nanomaterials for sensor applications. Reaction conditions—like acid versus base catalysis or humidity level—change the rates and structural details, a fact well known among those who scale up from bench to pilot plant. Instrument makers value its predictability, especially compared to more reactive silanes prone to premature curing or dangerous side reactions.
In the field, TPOrS goes by several names. Suppliers use tetrapropyl orthosilicate and tetrapropoxysilane interchangeably. Sometimes, catalog entries list silicon tetrapropoxide. It shows up in patent literature as Si(OPri)4, as well as under various trade names specific to the chemical supplier. Recognition is vital on the shop floor to avoid confusing it with the related tetraethyl or tetramethyl compounds, since similar containers often stand side by side in inventory. Long experience in chemical storage has shown the pain that comes from trusting shape or color code over cross-checked labeling; mistakes mess with both worker safety and research timelines.
Strict handling discipline matters with tetrapropylorthosilicate. The liquid reacts with water, giving off propanol, which builds pressure in sealed containers over time. Labs and plants store it under dry inert atmosphere, away from direct sunlight and ignition sources. Technicians suit up with goggles, gloves, and protective garments, since contact with skin or inhalation of vapors can irritate or, in some cases, cause more serious health issues. In case of a spill, teams deploy absorbent, non-flammable pads, followed by careful containerization for hazardous waste handling. National safety data sheets outline permissible exposure limits and recommend proper ventilation. Regulatory authorities audit sites for compliance, recognizing that even brief lapses breed long-term consequences, both to human health and environmental stewardship.
TPOrS finds use wherever the precise control of silica network formation is needed. It shapes advanced ceramics, supports designer catalysts, and enables scratch-resistant or anti-reflection films on optics. Electronics firms count on it for dielectric layer formation in microchips, while chemical engineers rely on it when crafting tailored pore structures for separation materials and adsorbents. Sol-gel processing benefits from its reactivity, helping labs and industries form fine powders, monoliths, or films for high-value end products. In research, it serves as a model compound for reaction mechanism studies—especially as teams push into nanoscale syntheses and hybrid materials for green energy or biotechnology.
R&D in the world of tetrapropylorthosilicate stretches from synthesis to end use. Chemists keep refining hydrolysis kinetics to lower temperatures, cut energy use, and maximize product quality. Interest in novel catalysts and binding agents often loops back to TPOrS as a surface modifier. Nanotech researchers lean on its predictable hydrolytic pathway for producing uniform particles, mesoporous frameworks, or composite coatings with customized functionality. Trials with alternative solvents, smarter process monitoring, and safer handling protocols push the boundaries further. Intellectual property filings document efforts to extend applications into drug delivery, controlled-release fertilizers, and next-generation photoresists in lithography—each use case putting stress on purity, stability, and process repeatability.
Scientists have devoted attention to understanding TPOrS’s effect on health and the environment. Early studies pinpointed its irritation potential, especially in concentrated vapor or spilled form. The main hazard flows from hydrolysis byproducts—propanol vapor, and, to a lesser extent, trace silanol intermediates, which can irritate respiratory passages and skin. Chronic exposure data remains limited, as TPOrS does not linger in the body; still, safety protocols demand strict containment and disposal, out of an abundance of caution. Modern laboratories seek to minimize fugitive emissions, and environmental audits flag any improper release into water courses given the risk of downstream toxicity. In large-scale operations, fume hoods and specialized detectors ensure workers stay protected and releases stay beneath regulatory limits. Continuous monitoring and new personal protective gear reflect both regulatory and cultural shifts, putting worker safety above expediency.
Looking ahead, TPOrS stands poised to support evolving needs in clean energy, electronics miniaturization, and sustainable manufacturing. Ongoing research hints at broader use in nanocomposite fabrication, especially where low-temperature processing and high-purity silica make the difference between successful and failed prototypes. As environmental rules tighten, expect a push toward greener synthesis, reduced solvent footprints, and recycling strategies for spent reagents. Lessons from existing marketplace disruptions—such as abrupt supply shortages driven by regulatory changes or new applications in emergent tech—remind everyone that diversification remains a safeguard. Investing in pilot-scale trials and reimagining transport systems for safer bulk movement will shape the next decade of production. The industry’s commitment to both innovation and safety should bring new opportunities for chemists, process engineers, and those looking to bridge laboratory science with everyday products.
Tetrapropylorthosilicate, known in labs as TPOrS or TPOS, pops up in a lot more places than most folks realize. As someone who’s worked with coatings and ceramics, I see this compound as one of those unsung heroes in high-performance materials. From supporting chemical reactions to holding up some of the glitziest tech out there, this molecule packs a punch far beyond its cryptic name.
TPOrS brings a lot to advanced materials. It serves as a source of silicon in sol-gel chemistry—a method for shaping materials at very precise scales. Many researchers count on TPOrS when making films and coatings meant to survive harsh wear or provide a barrier against moisture and corrosion. You see it in optical coating formulas, where it links up with other elements to form strong, clear glassy layers. High-quality camera lenses and certain medical devices count on these coatings, which depend heavily on the right silicon source.
Strong, lightweight, and chemically reliable silicon-based components don’t show up by accident. They’re the result of careful chemistry, where TPOrS acts as a key building block. In my experience, the best ceramics and silica gels trace their origins back to compounds like this. Going cheap or using impure sources leads to flaws and failures down the line. The reputation for reliability rests on choices made at the earliest synthetic stage.
Electronics makers have always hunted for ways to control materials at the smallest scale. TPOrS brings a certain edge. Used in producing silica-based films, it enables key elements in microelectronics and chip manufacture. Thin insulating films that protect circuits owe some of their best properties to silicon sources like TPOrS. If you’ve owned a phone, stepped in a hospital, or enjoyed a better-performing television, you’ve felt the impact of this chemistry.
Some applications focus less on the product, more on the process. Businesses use TPOrS as a cross-linking agent—a sort of chemical “glue”—that helps stick surfaces together at the molecular level. Certain adhesives and treatment products depend on this stickiness, keeping structures safe from heat, water, or other outside threats.
The compound also props up air and water purification systems. Silicon-based compounds catch contaminants and help filter industrial waste. That little molecular trick translates into cleaner environments, safer water, and healthier air in crowded cities. Many municipal and industrial systems would run less smoothly or at higher risk if they lacked reliable silicon chemistry.
Like many powerful industrial chemicals, TPOrS brings challenges when handling and disposal come up. Nobody enjoys reading safety sheets, but years around lab benches taught me that skipping this step invites mistakes. TPOrS—like many silicates—reacts with water and releases alcohols, which makes proper storage and personal protection essential for anyone nearby.
Waste handling and accidental releases raise flags. Without common-sense safeguards and trained workers, leaks could harm people or move into waterways. I’ve learned that regular training and labeling go a long way; accidents drop fast when teams stay vigilant and honestly communicate. There’s a real need for updated standards ensuring companies dispose of and use TPOrS responsibly, safeguarding both workers and neighborhoods.
Chemistry behind humble compounds often underpins the best of modern living. TPOrS keeps proving its worth, shaping products most people use every day. There’s an opportunity for companies to get ahead by investing in safe practices and transparent sourcing, building both trust and stronger products. In an era that prizes reliability and health, taking these extra steps benefits everyone, from the person behind the bench to the one holding a finished device.
Tetrapropylorthosilicate usually pops up in labs, foundries, and places that make high-performance ceramics or synthetics. Workers handling this compound deal with it as a colorless liquid, often poured into a batch of chemicals for coatings or catalysts. Not many outside industrial settings encounter it daily, but the risks matter for those who do.
Experience in chemical manufacturing teaches you that handling silicates means more than just wearing gloves. This particular silicate can irritate your skin, eyes, and lungs. Anyone who’s ever gotten a splash of solvent on bare skin knows it burns, and tetrapropylorthosilicate fits that description. It’s not only uncomfortable. Long-term or heavy exposure can cause lasting damage, sometimes leading to chronic breathing issues or skin disorders.
Studies show that tetrapropylorthosilicate causes acute irritation in animals and humans. Picture fumes in a closed room—without real ventilation or hood systems—making it hard to breathe and leaving your eyes watering. These aren’t just short-term annoyances; with repeated exposure, silica compounds can mess with lung tissue, even raising the risk of lasting respiratory problems over time. The breakdown products sometimes turn into propanol and orthosilicic acid, combining fire hazards with health hazards.
Investigators tracking chemical spills or leaks have documented cases where workers needed medical attention for coughing, headaches, and rashes. The US National Institute for Occupational Safety and Health (NIOSH) includes it on lists for hazardous workplace chemicals, recommending limits for time-weighted exposure and strict containment procedures.
Talking to people who’ve left the chemical industry, you see a common theme: not all plants hold up their end of the safety bargain. Lower-budget operations, especially in countries with looser labor laws, sometimes skip respirators or proper training. The risk grows if workers don’t even know what’s in the drum they’re pouring. In my own experience, I’ve seen skin blisters, breathing trouble, even trips to urgent care after accidental splashes that could have been avoided with more awareness and better gear.
Home use almost never comes up, since it’s an industrial scale chemical, but improper storage or disposal in communities near facilities creates an environmental hazard. A leaky barrel left outside can seep into the water table or release noxious fumes on hot days. Over time, regulators have stepped in to demand better handling, but enforcement varies.
Reducing risk starts with honest education. Employers in chemical plants have a duty to post clear hazard signage, run regular safety drills, and teach new hires the risks. Protective gear helps—goggles, gloves, and real respirator masks, not just a paper dust mask. Ventilation counts. Engineers should keep air moving where silicate vapors appear. Routine health checks for workers spot symptoms early, before problems get worse. Outside the plant, stricter waste handling and reporting requirements guard against accidental leaks.
Researchers and health experts keep studying tetrapropylorthosilicate for better treatment methods in case of exposure. The people most at risk deserve up-to-date information and practical support, not just complicated safety sheets.
For anyone working near dangerous silicates, health and safety don’t happen by accident. They come from learning, preparation, and sometimes a willingness to push supervisors for better protections.
Tetrapropylorthosilicate, known in many labs and factories as TPOS, comes with the chemical formula Si(OC3H7)4. To break that down, the silicon (Si) core binds to four propoxy groups. Each propoxy group stands for an –OC3H7 unit. So, the full molecule brings together four of these chains, hanging off one silicon, all tied up in an ester bond. This set-up changes the way silicon interacts with both humans and machines.
In labs and on factory floors, engineers and chemists have relied on silicon-based chemicals for decades. TP is not just a specialty product for a narrow field; it touches electronics, coatings, ceramics, and, for some, even the art of sol-gel chemistry. When making thin films or special glass, TPOS acts almost like a backbone, transforming liquid precursors into solid materials. I’ve seen researchers light up after setting up a reaction to make silica structures, knowing TPOS gives more control over properties like porosity or refractive index.
My own brush with tetrapropylorthosilicate came in graduate school while working on advanced glass coatings. I remember weighing out this clear liquid in a fume hood, the sharp sweet smell lingering even after the bottle closed. It gave the silica layers a smoother texture than other precursors, and our group noticed fewer defects. These details matter if you need optical precision or building blocks for tech that keeps shrinking year after year.
Having Si(OC3H7)4 means four propyl groups protect the silicon center. This chemical design brings more than stability. The carbon chains give the compound extra flexibility during certain reactions. In sol-gel processing, hydrolysis of these propyl groups releases alcohol (propanol) and lets silicon atoms crosslink into a network. Substituting a different alkoxy group (like ethoxy or methoxy) changes how fast the reaction moves, how much heat gets released, and even the kind of waste produced. By selecting propyl over methyl or ethyl, chemists slow down the reaction and have more time to shape the final material.
TPOS is not something to handle with bare hands. The liquid can cause skin and eye irritation and may release hazardous vapors in the air. Long-term exposure to some organosilicates can trigger health issues, especially if ventilation is poor or spills go unaddressed. Wearing nitrile gloves and working under a fume hood isn’t optional; it keeps accidents rare and damage to a minimum. Material safety data sheets spell this out clearly, and regular safety training stops small mistakes from turning into big emergencies.
For years, chemical plants have worked on greener ways to make and use compounds like TPOS. A few researchers now use renewable raw materials for the propyl groups or recycle waste solvents, cutting energy use and pollution. Companies adopting closed-loop production have an advantage, both for cost and community health. Regulators expect tighter controls too, with more oversight on how these chemicals get stored, shipped, and disposed.
Newer research points toward even safer alternatives that keep silicon’s unique benefits but lose the hazardous side effects. As institutions focus on both performance and safety, tetrapropylorthosilicate sits at a crossroads—offering huge benefits if applied thoughtfully and managed with care.
If you keep chemicals around for research, industrial production, or even routine quality testing, you eventually get asked about storage protocols. Tetrapropylorthosilicate, or TPOrS, isn’t some harmless solution you shove on a shelf and forget. Years spent in academic and industrial labs hammered home how practical habits—or, frankly, carelessness—directly impact everyone’s safety. Keep things simple: chemical storage must always come down to clear routines and respect for both the material and everyone handling it.
Tetrapropylorthosilicate is a colorless liquid, known for forming silica-based coatings and functioning as a crosslinking agent. What gets overlooked sometimes is its flammability and sensitivity to moisture. Poor habits, lax oversight, or basic ignorance mean unnecessary risks. One mistake can lead to warehouse fires, chemical burns, or expensive equipment damage. Safety rules never feel like busywork when you’ve seen a near-miss firsthand.
This chemical reacts with water, breaking down and releasing propanol. The byproducts alone cause headaches for anyone who assumes “dry” just means capping the bottle. In facilities I’ve worked at, a common failure was storing chemicals close to sinks, open containers, or areas with unpredictable humidity. Even air exposure from leaving bottles uncapped between pours led to contamination and reactions. Takeaways from real messes: always keep containers tightly sealed and stash them in dry places. A silica gel packet or two inside the storage cabinet does more than people give them credit for.
Like many organic liquids, TPOrS prefers stable, normal indoor temperatures. Direct sunlight or proximity to heating vents speeds up degradation and raises pressure inside containers. Overheated chemicals often expand, crack seals, or create internal pressure that results in leaks. I’ve seen temperature data loggers placed inside chemical rooms pay for themselves in a week, especially in climates where HVAC systems play games with ambient conditions. Always opt for temperature control rather than trusting luck.
Flammable liquids demand respect. Even a small spark or static charge turns carelessness into a costly disaster. Fire-resistant storage cabinets keep TPOrS away from ignition sources. In shops I worked in, keeping incompatible chemicals—like oxidizers or strong acids—separated by physical barriers or entire cabinets was a strict rule. Mixing storage without thinking ahead caused more than one emergency drill. Even replacing electrical outlets and light fixtures with explosion-proof models turned out worthwhile in the end, preventing accidents before they happened.
Misplaced confidence often leads to trouble. Marking every container, storage cabinet, and shelf keeps errors in check. In facilities with regular staff turnover, new workers rely on clear signage and updated logs to avoid repeating old mistakes. Real transparency only exists when everyone trusts the labeling system and records can be found in five seconds. A laminated emergency phone list on the wall got more use than half the dust-gathering SOP binders I’ve seen.
Build habits around training and a speak-up culture. Nobody should ever hesitate to call out strange smells or notice condensation on chemical containers. Frequent walkthroughs, quick retrainings, and clear signage keep people engaged. Training goes beyond first-time instructions—regular refreshers on chemical hazards show real commitment to safety. Simple systems like closed shelving, humidity indicators, and color-coded cabinets keep storage safer. They also speed up inspections, keeping workplace standards up to par and everyone out of harm’s way.
A lot of chemical compounds aren’t household names, yet their impact turns up in places most people don’t expect. Tetrapropylorthosilicate (TPOS) lands in this category. Folks working with coatings or electronics probably run into it the most, and they know it's not just another bottle on the shelf. It gives off fumes and turns dangerous if not respected. Trouble begins from the moment it leaves the drum, because those vapors sting the eyes, burn the skin, and scratch up the lungs. Anyone breathing them without care mistakes short-term ease for long-term harm.
I remember mixing small batches for lab tests, gloves always on, apron tied tight, mask hugging my face. I slipped once—just a fingertip tap—and felt the sting for hours. That one second proved how quickly things can go sideways.
Gloves matter—nitrile holds up, but thin latex won’t cut it. Splash-proof goggles stop unseen sprays from catching you off guard. Those who work in cramped storage rooms should know: small spills become clouds of fumes fast. Ordinary cotton shirts offer no shield. Lab coats block splashes, but sleeves and cuffs still let vapors through. I learned early to air out my workspace before and after, keeping the air moving so fumes wouldn’t settle.
You won’t find a good chemist popping open a bottle of TPOS in a closed room. Fume hoods make the process doable, pulling any floating vapors out of the worker’s breathing zone. Fresh air cuts down the risk of inhaling anything dangerous. Bottling up the compound and tucking it into a steel cabinet with a reliable seal keeps problems small. Plastic or glass alone won’t block leaks or vapors.
In some shops, I spotted containers stashed next to heat sources or stacked to save space. This practice tempts fate. TPOS, left too close to heat, reacts and pumps out toxic gas. Simple practice—put containers on lower shelves, far away from anything that generates warmth.
Mopping up spills changes everything. Those days, we relied on commercial absorbents designed for organosilicates. Paper towels never made the cut—they soak through too quickly and release more vapor. Dousing the spill with water seemed natural to rookies, but that mix releases even more heat and fumes. Sands or clay-based powders absorb without sparking fires or fumigating the room. Afterwards, we always shoveled the waste into tight-sealing drums, then tagged them for hazardous pickup.
Training never stops with a single lecture or label. Newcomers shadow veterans, learn which part of the routine is not up for shortcuts. Folks who asked questions stuck around; those who grew careless packed their lockers. Proper emergency eyewashes and showers hang close to the action. Even old hands know to run for the shower if splash strikes anywhere. Printed charts remind everyone what to do—nobody fumbles breaks or forgets to call for help.
No one wants to find out the danger the hard way. Respect for safety grows from stories and the scars old workers show. Research over recent years points out how organosilicates, like TPOS, can aggravate asthma and attack the nervous system. As more details fill in, routines will need to adapt again—new gloves, better filters, smarter storage. If the industry keeps sharing hard-earned lessons, fewer people will need to learn through mishaps, and the dangers will shrink, one shift at a time.
| Names | |
| Preferred IUPAC name | tetrapropyl silicate |
| Other names |
Tetrapropyl silicate Tetrapropyl orthosilicate Propyl silicate Silicic acid, tetrapropyl ester Tetrapropoxysilane |
| Pronunciation | /ˌtɛtrəˌprəʊpɪlˌɔːθəʊsɪˈlɪkeɪt/ |
| Identifiers | |
| CAS Number | 682-01-9 |
| Beilstein Reference | 2818736 |
| ChEBI | CHEBI:51227 |
| ChEMBL | CHEMBL3720545 |
| ChemSpider | 12313 |
| DrugBank | DB11266 |
| ECHA InfoCard | 227-045-5 |
| EC Number | 203-639-5 |
| Gmelin Reference | 78700 |
| KEGG | C18897 |
| MeSH | D013756 |
| PubChem CID | 66664 |
| RTECS number | VV9275000 |
| UNII | J4T7V8U80K |
| UN number | UN1992 |
| CompTox Dashboard (EPA) | DTXSID7014736 |
| Properties | |
| Chemical formula | C12H28O4Si |
| Molar mass | 284.52 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.943 g/mL at 25 °C (lit.) |
| Solubility in water | Insoluble |
| log P | 2.7 |
| Vapor pressure | 0.1 mmHg (20 °C) |
| Magnetic susceptibility (χ) | -87×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.408 |
| Viscosity | 3.5 mPa·s (25 °C) |
| Dipole moment | 2.05 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 540.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1462.65 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4896.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H226, H302, H319, H335 |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-2-NA |
| Flash point | Flash point: 102 °C |
| Autoignition temperature | 240°C |
| Lethal dose or concentration | LD50 Oral Rat 15000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 7380 mg/kg |
| NIOSH | TTT |
| REL (Recommended) | 100 ppm |
| IDLH (Immediate danger) | IDLH: 250 ppm |
| Related compounds | |
| Related compounds |
Tetraethyl orthosilicate Tetramethyl orthosilicate Tetraphenyl orthosilicate |