
Chemists started digging into organosilicon compounds in the early days of silicone research, somewhere around the 1930s and 40s. Methyltriethoxysilane (MTES) came along as researchers explored ways to bridge the versatility of organic chemistry with the heat resistance and flexibility of silicon. The need for water-repellent surfaces in electronics, construction, and coatings drove further innovation. By the 1950s, commercial paths had opened, mostly led by companies like Dow Corning and GE, who saw a market for insulators and weather-proof coatings. Laboratories developed routes using methanol and silicon tetrachloride, later refining synthesis to boost safety and performance. Over the years, the compound found its niche as both an additive and precursor for functional materials. Having worked with suppliers who have tracked the shifting landscape of silicones, I know that patents and production methods moved forward in tandem with growing environmental and industrial regulations.
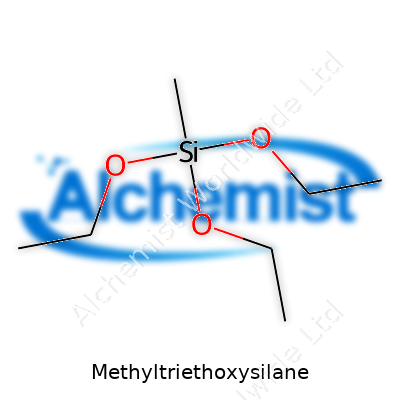
Methyltriethoxysilane stands out for its role as a silane coupling agent and surface modifier. The molecule features a single methyl group attached to a silicon atom, balanced by three ethoxy groups. On the ground, you see clear, colorless, sometimes sweet-smelling liquid in industrial drums labeled as MTES, with the CAS number 2031-67-6. You run into MTES when making water-resistant films, crosslinked polymers, or fancy self-cleaning glass for architecture. It shows up where materials must fight off humidity or bond to tough surfaces. MTES doesn’t usually turn heads, but it works behind the scenes in adhesives, glass coatings, optical parts, or as an intermediate in fancier silicone products. I’ve seen batches used for custom resins in research labs, and it delivers reliable modification without a complicated process or endless purification.
MTES sits as a low-viscosity liquid at room temperature. It boils above 140°C and flashes around 40°C, so the drum room needs ventilation and care. Density ranges close to 0.9 grams per cubic centimeter—a bit lighter than water—while the refractive index sits at about 1.38. The stuff floats on water but doesn’t stay put; if water touches it, you get slow, sometimes fizzy hydrolysis, leading to thick gels or films. MTES dissolves well in alcohol but separates from water because those siloxane bonds dislike moisture unless you push the reaction. Volatile enough to creep into safety discussions, but not so much that a few hours’ mishandling stink up a whole warehouse. In practice, the sharp, solvent-like smell tells you exactly when a drum or fugitive spill needs attention.
Manufacturers stamp labels with technical data like purity, which usually runs above 98%. Acid value, water content, and color bear watching since too much impurity gives unpredictable results in coatings and polymers. UN numbers, risk pictograms, and handling instructions matter for shipping and storage, especially since the liquid classifies as both flammable and irritating. From my experience, anyone working with drums signs off on storage below 30°C, tightly closed, in explosion-proof areas. Data sheets flag the importance of not breathing vapors, keeping away from ignition sources, using chemical goggles and nitrile gloves, and posting emergency contact numbers. Any casual storage, improper material transfer, or unlabeled leftovers bring real risks both for people and product integrity.
Industrial scale processes usually start with methylchlorosilane, reacting in a controlled setup with ethanol. Catalysts like hydrochloric acid drive the substitution, swapping out chlorine for the ethoxy groups. Temperature, ethanol-to-silane ratio, even stir rate, all influence the result. You want as little unreacted methylchlorosilane as possible to avoid corrosion, side products, and tough cleanup. The final batch needs careful distillation to reach high purity, and sometimes drying agents to mop up leftover ethanol or water. Small changes in process show up in product performance: inconsistent feeds can leave stubborn residues in pipes and reactors, or worse, taint the silane with acid traces that spoil downstream coatings. Having worked next to plant operators, I’ve seen that close monitoring trumps clever chemistry when chasing consistent yields.
MTES readily takes up water or even steam, hydrolyzing to build siloxane networks—essential for making films and crosslinked polymers. Under acid or base catalysis, the ethoxy groups start reacting, releasing ethanol and building Si–O–Si bonds that cure into tough materials. In coatings and sol-gel science, researchers tweak the hydrolysis rates or add co-monomers for hybrid structures. MTES can also branch into functional silanes by attacking the methyl group or swapping ethoxy for other leaving groups. Lab techs experiment with controlled hydrolysis to sculpt particle size for silica nanoparticles or trigger gelation in engineered glasses. From hands-on mixes in university labs, uneven mixing or misjudged pH can make or break a synthesis, leading to clumpy powders or sticky gels. You see firsthand how small formulation tweaks offer new uses for the old molecule.
Methyltriethoxysilane often appears as MTES, Trimethoxy(methyl)silane, or simply as “Silane, methyltriethoxy-”. Big producers ship it under brand names like Dow Corning Z-6383, Wacker M3, or ShinEtsu’s KBE-13. The naming changes just enough between suppliers to trip up new buyers, but the CAS number keeps things straight. Industry lingo sometimes drops the “triethoxy” for “-TES” or calls it “crosslinking agent” in product catalogs. Each supplier tends to print their own safety codes and material specification sheets, so cross-checking those details keeps mistakes at bay, especially during sourcing for critical applications.
Working with MTES requires good ventilation, chemical splash goggles, and nitrile or butyl gloves. A fume hood helps when pouring or blending. MTES catches fire near hot surfaces or open flames, and the fumes can irritate eyes, skin, and lungs. If you spill it on skin, wash fast with soap and water; if you breathe too much vapor, move to fresh air. Fire extinguishers—CO2 or dry chemical—should sit close, but water doesn’t help since MTES reacts and splatters. Waste needs special disposal, handled as hazardous chemical under codes set by EPA and OSHA standards. In my own lab experience, complacency leads to exposure incidents more often than lack of information. Adequate training, clear signage, and strict adherence to labeling keep teams safe, especially as new staff rotate through.
MTES finds work in construction sealants, waterproof coatings, and specialty glass. It acts as a crosslinker when curing silicone resins or adhesives, helping bind fillers to polymer backbones. Scientists in electronics rely on it to form insulating films resistant to weathering and moisture. Paint formulating rooms use MTES to help pigments stick to various substrates and boost water-repellency. Added to textiles, it creates anti-stain and anti-static finishes, making uniforms and upholstery more durable between washes. Having visited plants that coat automobile glass, I’ve seen MTES recipes improve clarity and scratch resistance, reducing consumer complaints. Engineers in energy sectors use it in cable insulation and weatherproof bushings, seeking long product life with minimal maintenance. The reach extends even to dental materials, where MTES modifies resins for precision fillings and crowns, blending clinical demands with chemistry advances.
Academic and industrial R&D teams continue exploring MTES for new surface treatments, nanostructured coatings, and membrane technologies. Scientists tweak reaction conditions to harness its reactivity for advanced composites or self-healing coatings. Rolling out novel catalysts pushes efficiency or sustainability, while green chemistry programs hunt for less toxic, renewable feedstocks. In my own collaboration with university researchers, efforts focused on adjusting hydrolysis to build porous networks for filtration or incorporate functional agents delivering unique electrical or optical traits. Computational models help predict performance in targeted environments, guiding experimental work. The drive for smarter, longer-lasting materials keeps MTES in the toolkit, and each research project seems to reveal a fresh capability, from marine antifouling to sensor technology.
Studies on MTES toxicity weigh occupational exposure against long-term risks. Acute contact can irritate mucous membranes, eyes, and skin, and if inhaled, the fumes may trigger coughing or headaches. Chronic exposure risks include organ effects, prompting regulators to set low permissible exposure limits in workplaces. Animal studies show limited bioaccumulation, since most MTES hydrolyzes and passes out, yet concerns about breakdown products, especially ethanol and silanols, keep toxicologists busy. Environmental researchers track its fate in water and soil, noting partial degradation and low persistence in most test setups, but persistent monitoring helps ensure safety standards stay up to date. Best-in-class hygiene, closed systems, and worker monitoring go a long way to cut real-world hazards.
MTES stands poised to play a bigger role in electronics, energy, and sustainable materials. The quest for more durable, repairable consumer products boosts demand for resilient surface treatments. MTES-based polymers evolve with the next wave of flexible electronics, photovoltaic modules, or specialty optics. Ongoing research investigates swapping in bio-derived or lower-toxicity alkoxy groups to align with stricter environmental rules. With green building standards guiding more projects, chemists invest in customizing MTES for safer, recyclable construction materials. I’ve seen innovation arise fastest where start-ups and established manufacturers share technical findings, lowering barriers to broader, safer uses. As societies value longevity, efficiency, and eco-friendliness, compounds like MTES transition from background chemicals to central ingredients in tomorrow’s technology.
I’ve seen the subtle influence of chemicals like methyltriethoxysilane in much of what surrounds us. This colorless liquid plays a quiet part in many industries, especially where strong and lasting bonds count. Construction teams rely on it in sealants, adhesives, and coatings because it delivers more than just a surface finish. It doesn’t just cover—it gets into the core of materials like concrete, glass, ceramics, and metals, making them resist water, mold, and wear over years of use.
Silane chemistry isn’t something I studied in school, but working in building renovation taught me why this chemical stands out. Add methyltriethoxysilane to a sealant and suddenly a brick wall holds up much better in the rain. This isn’t marketing talk; it’s science. Once it reacts with moisture, it sets off a process that anchors onto surfaces, making them more resistant to water and less prone to damage from humidity or frost. Research from building material manufacturers confirms that treated concrete stays intact longer, with fewer cracks and discoloration.
I’ve worked with caulks and silicones containing methyltriethoxysilane on kitchens and bathrooms, and learned quickly that mold doesn’t spread as easily. This isn’t just about nice-looking grout lines. The antimicrobial properties help prevent health issues caused by bacteria and fungal growth. Parents and people living with allergies benefit from the cleaner environment, and industry-backed safety reviews have found this to be one of the reasons professionals favor these treatments in hospitals and schools.
Electronics makers also count on methyltriethoxysilane. It acts as a surface modifier and crosslinking agent to improve the way plastics, glass, or metals stick in printed circuit boards and microchips. Smartphones, refrigerators, automotive parts—all these items gain reliability because of this improved bonding. Production yields go up and devices last longer.
Concerns about chemical exposure always come up. In my experience working with these products, wearing gloves and using proper ventilation helps a lot. Regulatory agencies in the US and Europe regulate its use, requiring clear labeling and safety data. Studies from the European Chemicals Agency confirm that, used correctly, the risks drop. Waste disposal and air quality count too. That’s why efforts have increased to use smaller amounts and switch to formulations that break down more easily in the environment.
Methyltriethoxysilane brings clear improvements in durability and hygiene in both household and industrial settings. For folks in construction, electronics, and home improvement, investing in products with safer silane chemistry can stretch the life of their work. Training workers on safe handling techniques keeps exposure risks low. Manufacturers have the chance to develop newer, safer alternatives—and some already do—to keep up with environmental standards and consumer demand for greener products.
Experience and independent research agree: this chemical isn’t just a technical ingredient. With the right care, it helps build spaces that are safer, cleaner, and built to last.
Methyltriethoxysilane shows up in labs and factories as a staple for making coatings, adhesives, and sealants. Its chemical structure means it reacts with water and makes ethanol and heat. Most people glance at the label and see flammable, but this chemical has more muscle than just fire risk. Leaked vapors can irritate your eyes and lungs almost as soon as you notice their sharp odor. Skin doesn’t do well with repeated exposure, either.
Anyone who’s spent time near strong chemicals knows the sting of splashes and accidental inhalation. Nitrile gloves last longer against alcohol-producing compounds like methyltriethoxysilane than latex, and lab coats or chemical-resistant aprons block surprises during transfers or spills. Eye protection isn’t optional—tight-fitting goggles stop those invisible vapor clouds from turning into emergency showers. If you’ve tried rinsing burning eyes under the tap, you’ll never skip goggles again.
The lungs need some love, too. Standard dust masks go nowhere with this stuff. You want a cartridge respirator designed for organic vapors. Not only do they keep you from hacking your way home after a spill, they also slow long-term lung damage. This chemical keeps environments dry to stay stable, so if you see condensation around a storage drum, double-check the seal. Leaks and moisture kick off chemical reactions fast.
Most fire stories with silanes follow the same script—one bad move, plume of vapor, spark—and the rest is sirens. Keeping methyltriethoxysilane away from open flames or electrical panels buys peace of mind. The ethanol it releases lights up almost instantly, so workers use spark-proof gear and tools without hesitation. In case of a spill, sand or vermiculite absorbs the liquid fast. Water just increases the risk, feeding the hydrolysis reaction and releasing even more ethanol. You’ll never see experienced handlers reaching for a water hose.
A proper ventilation system does more than keep odors low. Those fans whisk away vapor pockets before they turn up in the wrong corner of the room. A chemical fume hood, regularly serviced, keeps workers clear of airborne surprises. I saw one neglected fan go out during a reaction—ten minutes later, the whole room cleared out with coughing and headaches.
An inventory with regular checks for corrosion or leaks catches problems early. Small amber bottles help, since sunlight speeds up breakdown and makes containers go brittle. Workers always label containers, even for a small decant—one mistake sends people scrambling every time. Emergency eyewash and shower stations should never be blocked; if a spill hits skin or eyes, every second lost makes things worse.
Everyone who handles methyltriethoxysilane gets proper training before picking up a bottle. That familiarity saves lives because instinct kicks in when safety gets tested. Chemical manufacturers and the Safety Data Sheet spell out these needs clearly. Regular drills and open conversations about near-misses keep the team tuned in.
Safe handling of methyltriethoxysilane isn’t about long lists or endless rules. It comes from respecting the chemical, using the right gear, and leaning on proven routines. No room for shortcuts or guesswork. Facts and experience build that extra layer of protection, long before alarms ever sound.
If you’ve worked with industrial chemicals, you know safety depends on more than written guidelines. Methyltriethoxysilane, a silicon-based compound, sits high on the list for those who value both their products and their health. This chemical releases ethanol and methanol during hydrolysis—and that makes it a serious risk if someone stores it in a place where water or humidity sneak in. One small spill, and you’re left facing fumes you don’t want to breathe and surfaces you don’t want to touch without gloves.
Some people toss chemicals in a warehouse, screw the cap tight, and hope for the best. I’ve seen that approach turn dangerous overnight. Seals get loose, temperature shifts create pressure, and before anyone knows it, volatile vapors start to leak. Methyltriethoxysilane calls for more thoughtful precautions. A robust, airtight metal container keeps out moisture and oxygen. Plastic can break down, and glass leaves you one bad bump away from disaster.
Temperature control matters as well. Methyltriethoxysilane can break down and lose effectiveness when it sits in a hot environment or close to direct sunlight. More than that, heat can build pressure inside the container. Cool, shaded, well-ventilated storage minimizes all these risks. If you’ve ever worked a summer shift in an unventilated stockroom, you know how quickly fumes accumulate. Proper airflow saves people from accidental exposure.
In many plants and labs, I’ve watched the best safety habits build over time—and I’ve seen how lapses stack up to messes that require hazmat suits. Don’t just rely on hazard labels and hope everyone’s read the Safety Data Sheet. Clear signage around storage areas matters. Make sure every worker can spot a leaking or damaged drum in seconds, and train them to respond before a drip becomes a spill.
Store methyltriethoxysilane far from food, water, and incompatible chemicals. Acids and bases especially do not get along. One cross-contamination event can create a nasty reaction, and cleanup always costs more than prevention. Using secondary containment—solid, chemical-resistant trays under every container—turns a minor drip into a mess that’s easy to control. It’s the small decisions, like checking seals or keeping a drum off bare concrete, that protect everyone in the building.
Regulators expect full compliance with local, state, and national chemical storage requirements. Inspections can come without warning, and fines multiply if drums show up with crusted residue or if spill kits sit empty. More importantly, good storage practices show you care about coworkers’ safety and environmental health.
For anyone managing chemical stocks, a habit of clear records and regular audits goes a long way. Track expiration dates and use the oldest supply first. Keep detailed logs and verify them often. If your site recycles drums or containers, clean and inspect everything before new use.
Every workplace wants a clean safety record and operators who go home healthy. Storing methyltriethoxysilane calls for real-world awareness—vigilance, reliable containers, and a culture of care among staff. The payoff comes through steady work, fewer emergencies, and peace of mind for everyone on site.
Methyltriethoxysilane, with its chemical formula C7H18O3Si, stands as a mouthful for anyone not steeped in organosilicon chemistry. To make sense of this, think about ordinary products: sealants, adhesives, and some coatings. Each relies on additives and compounds for strength, flexibility, and resistance to wear. Methyltriethoxysilane lends a special hand in this space because it acts as a bridge between organic materials (like plastics) and inorganic surfaces (like glass or metal).
The practical value of methyltriethoxysilane sits beyond its formula. Most people see only the finished product, rarely the chemistry operating behind it. In adhesives, for instance, this compound brings resilience and reduces shrinkage over time. In coatings, it blocks out moisture—critical for stopping rust or damage on metal surfaces. Think of how double-glazed windows resist fogging or how electronics survive humid climates. Much of that protection depends on precise chemical engineering, where methyltriethoxysilane often plays a role.
Chemicals such as methyltriethoxysilane demand scrutiny, particularly in occupational settings. Its usage isn’t just a matter of mixing and pouring. Stable room conditions and safety gear step in for good reason; inhaling vapors or skin exposure can irritate, sometimes severely. Factories handling large volumes must follow established occupational standards, like those set by OSHA and REACH in the European Union. Splash goggles, good ventilation, and trained staff help prevent accidents.
When disposal comes up, the chemical’s impact stretches beyond the workplace. Pouring leftovers down drains or in open fields leads to soil and water contamination. Used thoughtfully, methyltriethoxysilane boosts product longevity and cuts down waste, but careless handling or disposal risks environmental damage. Industry leaders need robust waste treatment and clear instructions for end-users.
The reliability of products that use methyltriethoxysilane depends on chemistry that leaves nothing to guesswork. Manufacturers require tight quality checks, not just on finished goods but on each raw material shipment. Labs run tests like gas chromatography or spectroscopy to confirm purity. Impurities—even tiny amounts—can cause coatings to fail or adhesives to weaken. Consistent quality in supply keeps builders, auto makers, and electronics companies on stable ground, ensuring their products perform as promised.
It’s easy to overlook the role of a compound named methyltriethoxysilane in daily life. My own work in chemicals introduced me to just how interconnected things become: what seems like a simple sealant in a bathroom or the shatterproof panel on a subway train is anything but simple at the molecular level. Knowing the formula matters less to most users than the trust in safer, longer-lasting, and environmentally responsible products. But it all starts with getting the chemistry right—down to every last atom in C7H18O3Si.
Methyltriethoxysilane gets used in things like paints, coatings, and adhesives. It helps materials stick to surfaces, and you find it in industrial settings everywhere. With that much exposure, it makes sense to look at what it does to living things and the planet.
Most people never touch pure methyltriethoxysilane, but workers in labs or factories do. That’s where real risks live. Even without direct skin contact, fumes can sneak in through lungs. I once visited a facility using this compound for glass treatment. Just walking through, I caught a whiff of something sharp. The folks working there wore proper gear, and I realized why—safety depended on it. Studies show that breathing in even small doses can cause nose and throat irritation. U.S. government data and material safety data sheets say eye and skin contact runs the risk of burns or rashes. Long-term exposure hasn’t been studied enough, but a few case reports mention headaches and breathing issues. NIOSH and OSHA both consider it a chemical where caution means more than just posting a warning sign.
Spills or leaks matter more than most people guess. Methyltriethoxysilane breaks down in water, making ethanol and other siloxanes. That doesn’t sound too bad, but siloxanes hang around. Fish don’t handle these by-products well, and some local rivers near big plants have suffered from the effects. It’s not just about what gets poured out—runoff after a rainstorm can carry a surprise load right into drains or groundwater. Once there, it doesn’t just disappear. If handled poorly, the breakdown products build up and stress aquatic plants and insects. I remember reading a field study from Europe in siloxane-contaminated rivers: more algae, fewer healthy fish, and sharp falls in water insect counts right below industrial outlets.
Strict guidelines shape how to store and use the stuff. Companies who value worker health tell their teams to suit up, use goggles, and keep tanks sealed. Engineering controls, like well-designed ventilation, work best in keeping vapors from drifting. The chemical industry points to "closed systems" where no open air meets the product, which drops the chance of accidental exposure. Real progress happens when these controls get reviewed and improved, not just installed and ignored. In my own circle, I know safety specialists who audit these measures twice a year because once isn’t enough.
It’s not enough to leave things up to regulation. Manufacturers need to keep training fresh, submit to surprise inspections, and use third-party reviews. Waste treatment matters too. Proper disposal prevents leftovers from finding a shortcut to farmland or water. Some countries, like Germany and Japan, have ramped up rules on siloxane disposal. The rest of the world could learn from those models. I believe industry transparency is the key; you shouldn’t have to dig for safety records or rely on outdated law. Real accountability starts with honest reporting, not just compliance paperwork.
Methyltriethoxysilane isn’t the scariest chemical out there, but it deserves respect. With direct skin and vapor risks for workers, plus a chance to hurt waterways, it calls for careful handling and oversight. Smart management and open dialogue mark the difference between safe jobs and reckless shortcuts. In my experience, companies that treat risk as a daily challenge—not a box to tick—keep both their people and their surroundings healthier.
| Names | |
| Preferred IUPAC name | Triethoxy(methyl)silane |
| Other names |
Triethoxymethylsilane Methyltriethoxysilane MTES |
| Pronunciation | /ˌmɛθɪlˌtraɪˌɛθɒksoʊˈsaɪleɪn/ |
| Identifiers | |
| CAS Number | 2031-67-6 |
| Beilstein Reference | 1367039 |
| ChEBI | CHEBI:85264 |
| ChEMBL | CHEMBL1900052 |
| ChemSpider | 86112 |
| DrugBank | DB16663 |
| ECHA InfoCard | 100.039.528 |
| EC Number | 203-635-6 |
| Gmelin Reference | 52948 |
| KEGG | C19230 |
| MeSH | D016592 |
| PubChem CID | 66248 |
| RTECS number | VV4950000 |
| UNII | K1CFA43F40 |
| UN number | UN1992 |
| CompTox Dashboard (EPA) | DTXSID2020587 |
| Properties | |
| Chemical formula | C7H18O3Si |
| Molar mass | 178.29 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Pungent |
| Density | 0.893 g/mL at 25 °C (lit.) |
| Solubility in water | Insoluble |
| log P | 0.6 |
| Vapor pressure | 1.2 hPa (20 °C) |
| Acidity (pKa) | Acidity (pKa): ~18.7 |
| Basicity (pKb) | pKb > 7 |
| Magnetic susceptibility (χ) | -7.0E-6 cm³/mol |
| Refractive index (nD) | 1.377 |
| Viscosity | 1 mPa.s (25°C) |
| Dipole moment | 2.20 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 294.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -520 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1795 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H302, H332, H319, H335 |
| Precautionary statements | P210, P261, P280, P301+P312, P303+P361+P353, P305+P351+P338, P337+P313, P370+P378 |
| Flash point | 50 °C (122 °F) |
| Autoignition temperature | 254°C |
| Explosive limits | Explosive limits: 1.3% (LEL) - 15.0% (UEL) |
| Lethal dose or concentration | LD50 Oral Rat 1700 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 7060 mg/kg |
| NIOSH | GVG30000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Methyltriethoxysilane: "200 ppm (TWA) |
| REL (Recommended) | 50 ppm |
| IDLH (Immediate danger) | 850 ppm |
| Related compounds | |
| Related compounds |
Methyltrimethoxysilane Vinyltriethoxysilane Ethyltriethoxysilane Phenyltriethoxysilane Tetramethoxysilane Tetraethoxysilane |