
Trimethylisopropenoxysilane entered the chemical scene during an era hungry for innovative organosilicon compounds. Its story weaves through decades when the silicon industry craved new reagents for tailoring advanced polymers and surface coatings. Chemists, experimenting with silicon-containing monomers, noticed the unique combination of isopropenoxy and trimethylsilyl groups. Early records from the mid-20th century show patent filings as researchers began viewing this compound as more than a curiosity. Silicon chemistry grew fast, much like the information age blossomed alongside computing—Trimethylisopropenoxysilane became one of those bridge molecules opening pathways between organic and inorganic domains, dovetailing with needs in microelectronics and specialized polymer science.
Trimethylisopropenoxysilane always draws attention among silylating agents. Its structure—trimethylsilyl group tethered to an isopropenoxy unit—carries a distinct ability to modify surfaces, work as a chemical intermediate, and bind functionally in tough reaction environments. Silicon-based coatings, adhesives, sealants, and lubricants often lean on this material to tune adhesion or flexibility. I recall a colleague, deep in the world of specialty films, who pointed out the keen difference this compound made versus traditional trialkoxysilanes, especially when surface gloss and wear resistance mattered. Companies stock it for very specific needs; it rarely sits on back shelves, instead finding real-world uses where others stall.
In the lab, Trimethylisopropenoxysilane appears as a clear, colorless liquid with a distinct, somewhat sweet, ether-like smell. Its boiling point lands around 100°C, which stays within handling range for most synthetic chemists. The compound holds a density near 0.78 g/cm3, lighter than water, so it floats in most mixtures—useful for separation. Its vapor can ignite at room temperature, so sources of static or open flame need strict control. This silane dissolves well in many organic solvents, dodging water entirely due to quick hydrolysis. Chemically, the isopropenoxy group lends reactivity for further modifications, but the backbone resists breakdown under normal acid-free conditions.
Manufacturers ship Trimethylisopropenoxysilane in metal cans or glass bottles, sealed against moisture. On every label, users find the CAS number (i.e., 18216-70-3), purity ratings often pushing 98% or higher, and basic hazard warnings. Detailed safety data sheets dive into flash point numbers (usually 7°C), handling instructions, and shelf-life guidance—my experience says unopened containers keep their punch for over a year, but once air touches them, degradation picks up speed. Proper labeling includes batch numbers for traceability, with transport regulated under flammable liquid statutes. Plant engineers check these specifications shut before clearing materials for use.
Producers follow a fairly direct route: they react trimethylchlorosilane with potassium isopropenoxide at low temperatures under dry inert gas. The process brings together the two components, then distills off byproducts such as potassium chloride. From lab-scale glassware to industrial reactors, water sensitivity stays front and center. Chemists flush lines, dry vessels, then monitor distillation closely; even tiny leaks will ruin yields. In my own grad-student days, I watched a senior scientist lose half a batch from just one cracked ground glass joint. The final distillate comes pure, but every step exposes the operator to volatile fumes, so strict protocols always rule the day.
Trimethylisopropenoxysilane reacts swiftly with water, alcohols, and acids by cleaving the isopropenoxy group, freeing silanols in situ. It serves as a silyl donor in many protection-deprotection reactions, siding up to alcohols and amines to create robust silyl ethers and silyl amines. Polysiloxane formation leans on it to control crosslinking density—key for tailoring rubber industries and specialty elastomers. The isopropenoxy group’s double bond offers a portal for further functionalization, such as hydrosilylation. Careful tweaking of reaction conditions unlocks selective modifications, so researchers build out complex silicon networks without relying solely on traditional silicon-oxygen bonds.
Chemists and industrial buyers run across this chemical under several names. Some lists show it as 1-Trimethylsiloxy-1-propene, others prefer Trimethyl(1-propenyloxy)silane. Trade formulations or technical catalogs might slip in “TMS-propene ether” or abbreviate to TMIPOS. It’s a land of many labels, so veterans double-check CAS numbers, especially negotiating with global suppliers. This prevents costly confusion with similar silylating agents that don’t share the same reaction profiles.
Operators need strict safeguards working with Trimethylisopropenoxysilane. The fume is flammable, the liquid volatile, and accidental splashes burn soft tissue. I learned early—never pipette this stuff by mouth; always vent hoods and splash shields. Eyes, gloves, and flame-retardant lab coats stay mandatory. Air monitors check for vapors, and double egress out of reaction rooms stays policy, mainly to avoid rushes during accidental spills. Factories set up dedicated silane rooms with explosion-proof light switches. Safety data shows how inhalation irritates airways, while even brief skin contact leaves burns or redness. Training programs include regular drills on spill cleanup, fire suppression, and first aid, making sure every team member knows the fast way out and how to seal containers.
Trimethylisopropenoxysilane keeps popping up in places needing surface priming, cross-linking, or hydrophobic coatings. In the semiconductor industry, engineers use it for modifying wafer surfaces, setting up etching steps or photoresist adhesion. Paint companies toss it into formulations for anti-fouling and scratch-resistant layers. In adhesives, it strengthens bonds under wet or salt exposure. I’ve seen polymer chemists use it for bringing just the right flexibility to synthetic rubbers, fine-tuning seals that handle aggressive solvents. Electronic encapsulation, specialty lubricants, and even dental impression materials all benefit from its unique profile—few chemicals swing between roles so easily. Once you dig into technical literature, its name threads through patents across disparate fields, especially any process depending on controlled silicon-oxygen architectures.
Universities keep digging into new reactions involving Trimethylisopropenoxysilane. In organic synthesis, teams search for more efficient pathways for silyl protecting group installation that minimize waste and boost selectivity. Environmental labs experiment with this reagent as a tool for producing new water-repellent or nonstick surfaces. In nanotechnology, researchers look at assembling silicon-based cages and frameworks for drug delivery or microfabrication—always seeking higher surface control and stability. My own research circles talk about this chemical in breathless tones when methods lack precision; it’s often part of solving those thorny problems where lesser silylating agents create side products or drag down efficiency.
Toxicologists classify Trimethylisopropenoxysilane as a moderate hazard. Animal studies and cell cultures show respiratory and dermal irritation at low exposures; larger doses lead to systemic effects, especially involving kidney and liver stress. Inhalation can cause dizziness, nausea, and longer-term lung function changes. The hydrolysis products, mainly methanol and silanols, add their own risk. Waste management addresses these by channeling spent solutions into controlled incineration. Regulatory agencies keep adjusting occupational limits, recently tightening permissible exposure. Researchers keep testing for chronic impacts. Lab workers talk numbers like LD50 and NOAEL in safety meetings, but field experience sets the bottom line—never take organosilanes lightly, and always treat symptoms quickly.
Trimethylisopropenoxysilane’s future ties directly to the drive for advanced materials. As electronics keep shrinking, demand rises for precise, reworkable surface chemistries. New green chemistry initiatives pressure manufacturers to cut waste and develop recyclable silicon-based products—more efficient syntheses using this reagent may hold answers there. The medical device field keeps testing hydrophobic surface treatments for implants or catheters, hoping for longer lifespans and fewer infections. Advanced composites in aerospace, automotive, or renewable energy keep searching for durable, lightweight coatings. Interest in 3D printing with silicon-rich resins grows each year. If chemical engineers and synthetic chemists can tame production costs and dial in safer handling, this molecule promises a wider reach in specialty manufacturing and sustainable technologies.
Trimethylisopropenoxysilane doesn’t show up in headlines, but it’s been a steady contributor behind the scenes. This compound, with its tongue-twisting name, belongs to a family of chemicals called silanes—each bringing something special to the worlds of coatings, plastics, and electronics.
I’ve worked in labs where finding a way to keep moisture out of a reaction meant the difference between a pass and a spoiled batch. Silanes, particularly trimethylisopropenoxysilane, offer unique shelter from the chaotic world of water molecules. Their molecular structure makes them highly reactive with hydroxyl groups, which gives companies a convenient way to convert surfaces from water-loving to water-hating. That property plays out in products like protective coatings or advanced adhesives.
Electronics manufacturing, especially in semiconductors, has no room for error. Trimethylisopropenoxysilane brings clean lines and precise surfaces. Chip houses add it to silanization steps—tiny wires, glass, or silicon wafers get a coating, almost like a raincoat, so they don’t stick or short. This chemical helps those delicate surfaces stay reliable, unreactive, and easy to handle from fab to packaging.
Glass and ceramics benefit in other ways. It surprises some folks, but glass isn’t as smooth as it looks under a microscope. It’s pitted and imperfect, which is a problem for optical work or specialty mirrors. With this silane, glass surfaces become much less likely to suck up moisture or shed their glossy look. Specialty glassware in labs, camera lenses, and even architectural windows get a boost from these coatings.
The arms race to make tougher and more weather-resistant plastics never stops. Trimethylisopropenoxysilane works as a modifier in plastics processing. It reacts through its silane group, sticking to one part of a polymer chain while the “trimethyl” cap blocks unwanted reactions. The result is often a slicker, tougher finish. Automotive and construction sectors use it to make sealants that cling to glass and metal for years, rather than peeling off after a single hot summer.
Silane chemistry gives a lot, but handling comes with trade-offs. My gloves never lasted long in the presence of reactive silanes—safety goggles became an extra pair of eyes. Trimethylisopropenoxysilane can irritate skin or eyes and needs careful storage away from water or air. Employers owe it to their teams to invest in the right PPE and invest in engineering controls to keep vapor concentrations down. In the US, the EPA and OSHA set clear guidelines, though the fast pace of new uses sometimes makes catching up tough.
The chemical industry faces rising demand to reduce emissions and improve recyclability. Down the road, greener alternatives and closed-loop systems may limit silane use. Innovations in plasma and vapor-phase processing show promise for lowering the chemical load while keeping the same strong performance.
Trimethylisopropenoxysilane stays out of the news, but its presence is everywhere. From electronics assembly to spray coatings, it streamlines work, stops problems before they start, and brings value to industries that prize reliability. Those of us who understand its promise also recognize the importance of handling it right and pushing for a future with smarter, safer chemicals.
My early days in a small research lab offered a quick lesson: chemistry doesn’t forgive mistakes. Take Trimethylisopropenoxysilane, for example. This mouthful of a compound packs flammable, volatile, and reactive properties. The minute you open a bottle, the vapor hits fast. That sharp smell serves as a loud warning—one sniff tells you this is not a substance to handle without care.
It only takes one careless mistake. I’ve seen a coworker touch a contaminated bench, only to feel skin irritate in minutes. Gloves offer a basic barrier, but not all types block every chemical. Nitrile or neoprene gloves outlast the quick-breaking latex ones, and anyone who grabs a bottle with bare hands invites a painful lesson. Spills creep, stains spread, and sometimes stings last. Soap and water help after the fact, but prevention always works better.
Pouring or measuring produces fine splashes. I never walk into a room with an uncapped bottle without my goggles snug. A face shield adds another level when volume increases. One time, I saw a drop hit a cheek, burning red almost instantly. Eyes suffer much faster. Permanent damage comes quick—eye wash stations only reduce the harm, not erase it. I keep goggles on long after cleaning up.
Trimethylisopropenoxysilane isn’t forgiving if you let fumes build up. Every lab worth its salt uses fume hoods, not open desks. Modern hoods trap fumes and block exposure. But not everyone gets ideal equipment. Small companies and old classrooms skip ventilation, risking nosebleeds, headaches, or much worse. Respirators step up protection, but a working hood removes the source—no mask offers that peace of mind.
Flammability stands as one of the main dangers. Heat sources, static sparks, or even a lighted cigarette lead to disaster. One careless sweep with a hotplate or static-prone fleece jacket could change everything in seconds. Keeping this material away from open flames isn’t a nice-to-have rule. Regular checks on storage—dry, cool, tight-sealed bottles kept away from sunlight—make a real difference. Years in the business taught me that good labels, with clear warnings, mean fewer accidents.
Even with the best habits, spills still happen. Having absorbent pads, correct neutralizers, and a dedicated waste bin close at hand means nobody scrambles in the moment. Staff or students always need a quick drill—how to clear the room, where the exit sits, how to call for help. The labs that put safety gear where everyone can see it have the fewest incidents.
People working with hazardous chemicals need more than a printed sheet of rules. Shared stories, visible scars, even short case studies keep lessons real. We never outgrow the need to check over material safety data sheets, even after years on the job. The best labs don’t trust memory or ignore small leaks. No shortcut ever replaced a focused, respectful approach to chemicals like Trimethylisopropenoxysilane.
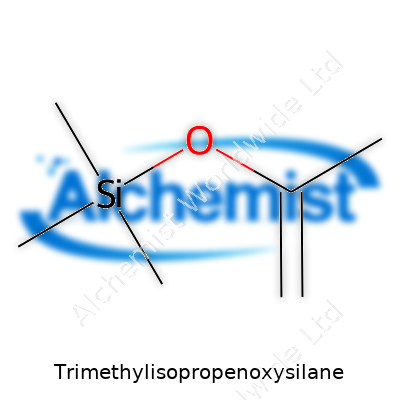
Trimethylisopropenoxysilane comes with a chemical formula of C6H14OSi. To put it simply, the structure includes a silicon atom attached to three methyl groups and one isopropenoxy group. Picture it as Si bonded to three CH3 groups and a longer chain that features an isopropenoxy bridge—a layout that gives the molecule its name and strong reactivity.
The core structure looks like this: the silicon (Si) atom stands at the center, surrounded by three methyl groups (–CH3), which bring stability and reduce the likelihood of unwanted side reactions. The final connection is the isopropenoxy group (–O–C(CH3)=CH2), a side chain known to bring unique chemical properties. This small change in the silane backbone opens up big opportunities in surface science and silicon-based chemistry.
From years of handling specialty silanes in the lab, I can confirm these chemical markers define reactivity and dictate how reliably the compound works in different settings. On the bench, the isopropenoxy group delivers a convenient spot for further reactions. You get a reliable precursor for the creation of protective silicon-based layers or for grafting onto glass fibers. The three methyl groups pull their weight by making the whole molecule more resistant to water, which slows hydrolysis and offers much-needed shelf life in moisture-rich environments. I've seen silanes like this one prolong the life of chemical precursors that usually degrade quickly in the presence of air or slight humidity.
As a chemist, it's always tempting to go straight for convenience or price. Yet, knowing the actual molecular structure helps avoid costly mistakes. Trimethylisopropenoxysilane reacts selectively—a plus for folks wanting to set up robust silicon-oxygen bonds during surface modification projects. For instance, coating glass with this silane can help change how water or organic solvents interact with the material. Its hydrophobic methyl tails stick out, discouraging pooling and messiness when liquids hit the treated surface.
Commercial manufacturers choose trimethylisopropenoxysilane as a coupling agent for composites or as a primer in electronics manufacturing. According to data from regulatory groups, the use of silanes in microelectronics grew by 18% over the last five years. Its backbone stability gives a big boost to the long-term reliability of circuit boards. In adhesives, the molecule keeps different substances bonded together, even if temperatures swing or moisture levels shift.
Working with this compound requires a careful approach. It isn't the most hazardous member of the silane group, yet inhalation or direct contact can irritate sensitive skin. Anyone working with it should suit up with gloves, goggles and decent ventilation. Based on guidance from experienced safety officers, ensuring well-sealed containers and preventive spill measures always tops the list before beginning a synthesis.
Not every lab or workshop needs high-tech handling systems to stay safe. Even basic fume hoods, tight closures and labeled containers make mistakes unlikely. I learned from early mistakes that spills—no matter how small—leave less time for chemistry and more for damage control. Hydrolysis of trimethylisopropenoxysilane produces methanol and other volatile byproducts, so tracking ventilation and monitoring exposure levels stays critical, especially for people working several hours a day with silanes.
Looking toward a sustainable future means choosing silanes that offer strong performance with lower environmental load. By using only what’s needed and recycling unreacted material when possible, users can cut costs and waste. If companies adopt closed systems for silane delivery, then less vapor escapes, and both staff and the environment fare better in the long haul.
Trimethylisopropenoxysilane brings a good mix of durability, selectivity and ease of use that makes it a favorite for both cutting-edge labs and industrial giants. Its straightforward structure lies at the center of its resilience and flexibility, showing how fine-tuning a molecule’s side chains unlocks fresh potential in everything from electronics to materials science.
Trimethylisopropenoxysilane doesn’t take up headlining space outside chemistry circles, yet anyone handling it understands the value of getting its storage right. From my own days in research labs, I’ve learned that skipping over proper chemical management invites way more trouble than anyone needs. Spills, fires, breath-stealing fumes—all true risks with simple slip-ups. This organosilicon compound delivers flexibility in industrial coatings and electronics, but its volatile nature also brings real headaches.
A handful of facts speak for themselves: it catches fire easily, forms explosive mixtures with air, and reacts with even modest moisture. And if you’ve ever uncapped a container near a muggy sink and watched fumes sizzle out, you don’t forget the lesson. It’s listed under hazardous substances in global regulations for good reason. The flash point hits below room temperature, encouraging strict respect for even minor spills or vapor escapes.
Over the years, I’ve seen people improvise storage with recycled bottles or sketchy cabinets. Every shortcut ups the risk. Dedicated, properly labeled containers—glass or specific plastics tested for this chemical—set the basic standard. Always go for a tight-fitting cap. Exposure to air or humidity even for a few minutes can trigger dangerous reactions or degrade the product.
A flameproof metal storage cabinet with clear signage and no clutter on top keeps things straightforward. I once worked for a place that tried using ordinary wooden shelves. After one bottle cracked and released vapors, the lesson hit home—always choose a cabinet rated for flammable chemicals. Keep Trimethylisopropenoxysilane far from oxidizers, acids, or bases. Mixing even accidental drops led to a lab evacuation one autumn afternoon. So, cross-check chemical compatibility before stacking containers.
Heat speeds up decomposition and makes flammable vapors more likely. Cool, dry, out-of-the-way spaces make the best homes for volatile organosilanes. Some facilities run small explosion-proof refrigerators just for chemicals like this. Humility goes a long way; thinking, “one day won’t hurt” tempts fate more than most realize.
Good ventilation saves headaches—literally. Well-designed chemical storage areas usually have mechanical ventilation or fume extraction. Once in an older school, an unvented closet led to headaches and mystery smells. Newer systems cut those risks. If budgets run tight, at minimum store containers near open windows or exhaust fans, but never in spaces shared with food or personal items.
No storage system covers for basic mistakes made by untrained users. Reminders posted in the room about wearing gloves and goggles, as well as specific emergency steps, lock safety into routine. Fire extinguishers rated for chemical fires should stay within arm’s reach. I remember one drill where someone doused burning solvent with water, making things worse. Fire training and spill response plans should sit right alongside inventory logs.
Tight logs keep surprises to a minimum. Tracking shelf-life matters because this silane breaks down over time, even in ideal storage. Expired product ends up back in hazardous waste streams, leaking money and labor. Regular checks for bottle conditions, capped seals, or crusty residue help spot problems before they grow. Experienced lab managers never leave these decisions to chance or guesswork.
Trimethylisopropenoxysilane pops up in lab supply catalogs and paint booths alike. You’ll find it under specialty chemical names, mostly used as a crosslinking agent or a protective additive in surface coatings. I’ve had the chance to work beside coatings chemists who depend on this silane to enhance resistance and grip in their formulations. Mixing in a reactive silane always turns into a bit of a gamble, though, unless you know your ingredients from top to bottom. Unexpected chemical reactions don’t just mess up a batch—they can gum up valves, spike operating costs, and carry a safety risk for anyone nearby.
This is not your run-of-the-mill ingredient that plays well anywhere. As soon as moisture sneaks in, trimethylisopropenoxysilane breaks down, releasing isopropenol and leaving highly reactive silanols behind. So, water-based systems and humid storage areas often become sources of headaches. Even a tiny splash or a slip in humidity control can start a messy reaction, creating byproducts that don’t belong in your mix.
Many shops stick with common solvents like toluene or xylene to help coat formulations blend. This silane handles those with fewer surprises. On the flip side, mixing with strong acids or strong bases usually backfires. The molecule quickly falls apart, sometimes releasing flammable or toxic fumes. I’ve seen this play out in a bench-top experiment: the mixture heated up, fizzed, and made a sticky mess.
Even amines, which chemists sometimes pick to tweak surface bonds or improve adhesion, can hit trouble. Reacting with trimethylisopropenoxysilane shoots off heat, creating stuff that clogs up spray guns and hoses. In a large-scale setting, runaway reactions put a strain on ventilation systems and can put workers at risk of exposure or injury. The lessons here cost time and money, not to mention peace of mind.
Even the best chemical intuition benefits from a good look at available data. Safety Data Sheets lay out known incompatibilities—trimethylisopropenoxysilane ranks as sensitive to both water and oxidizers. Safety authorities like NIOSH and OSHA outline what’s at stake during blending and storage. If it splashes on skin or gets breathed in, that’s where personal protective equipment makes a difference. Fume hoods and gloves aren’t just recommendations here; they count as essentials.
Whether you’re formulating paints or adhesives, bringing in a new silane compound takes preparation. Storage involves strict seals and dry air. Some shops add desiccants in shipment containers or double up on moisture indicators. Quality control teams I’ve known stick with small trial batches, tracking for any haze, cloudiness, or heat spurts. These hands-on checks catch trouble before a full production run risks bigger losses.
I’ve seen teams get the best results by involving a professional chemist from square one. Running spot checks, scanning batch logs, and using in-house compatibility charts helps sort out what blends without a hitch. Labs credit a lot of success to collaboration, keeping open lines between purchasing, safety, and production. Some operations build digital reminder systems so nobody accidentally mixes incompatible drums.
Above all, people involved in this work stress teamwork and continuous learning. Experienced hands remember near-misses and pass those stories along—a key part of company culture that balances productivity with safety. Mixing smart and watching for early warning signs go further than any rule book. For anyone handling trimethylisopropenoxysilane, experience gets the last word on blending safely.

| Names | |
| Preferred IUPAC name | Trimethoxy(2-methylprop-1-en-1-yl)silane |
| Other names |
1-Methoxy-1-methylethyl)trimethylsilane Isopropenoxytrimethylsilane Trimethyl(1-methoxy-1-methylethoxy)silane Trimethyl(1-methoxy-1-methylethenyloxy)silane |
| Pronunciation | /traɪˌmɛθɪlˌaɪsəˌproʊˈpiːnɒksiˌsaɪleɪn/ |
| Identifiers | |
| CAS Number | 34396-03-7 |
| Beilstein Reference | 1721442 |
| ChEBI | CHEBI:87136 |
| ChEMBL | CHEMBL1567201 |
| ChemSpider | 157368 |
| DrugBank | DB11273 |
| ECHA InfoCard | echa.europa.eu/information-on-chemicals/infocards/100.014.173 |
| EC Number | 245-366-4 |
| Gmelin Reference | 55808 |
| KEGG | C18529 |
| MeSH | D015589 |
| PubChem CID | 11627221 |
| RTECS number | VV5950000 |
| UNII | 5O7M0J211Z |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID20211914 |
| Properties | |
| Chemical formula | C6H16OSi |
| Molar mass | 188.36 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.824 g/mL at 25 °C |
| Solubility in water | Insoluble |
| log P | 0.9 |
| Vapor pressure | 3.8 hPa (20 °C) |
| Acidity (pKa) | 17.9 |
| Basicity (pKb) | Trimethylisopropenoxysilane has no measurable basicity (pKb) as it is not a basic compound. |
| Magnetic susceptibility (χ) | -6.44×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.384 |
| Viscosity | 2 cP (25 °C) |
| Dipole moment | 1.177 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 324.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -386.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4562 kJ/mol |
| Pharmacology | |
| ATC code | V04CX |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H319, H315, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P264, P280, P301+P312, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-4-1-W |
| Flash point | 49 °F (9.4 °C) |
| Autoignition temperature | 230 °C |
| Explosive limits | Explosive limits: 1.1–7.0% |
| Lethal dose or concentration | LD50 (Oral, Rat): > 2000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2300 mg/kg |
| NIOSH | GGG |
| PEL (Permissible) | PEL: Not established |
| Related compounds | |
| Related compounds |
Trimethylsilyl chloride Trimethylsilyl trifluoromethanesulfonate Trimethylsilyl cyanide Triethoxysilane Trimethylmethoxysilane |