
In the early days of organosilicon chemistry, researchers focused on bridging the gap between organic and inorganic worlds. Vinyltriacetoxysilane came into the spotlight during the commercial push for improved adhesives and sealants, right around the expansion of plastics and polymers in the 1960s and 70s. Laboratories across Europe and America began pressing for coupling agents that could help plastics stick better to glass, metals, or ceramics. Vinyltriacetoxysilane arrived on the scene with its unique vinyl functionality and acetoxy leaving groups, shifting how chemists designed interfaces. Through the decades, industrial production scaled up, meeting the demands of construction, electronics, and coating industries. Often, conversations about making stronger composites or more weather-resistant sealants included this silane as a key additive.
Vinyltriacetoxysilane falls under the silane coupling agents category, sporting a vinyl group at one end and three acetoxy groups attached to a silicon atom. This gives it the ability to bond organic materials, like polymer chains, to inorganic surfaces. In practice, this silane boosts adhesion, improves wetting, and helps resins cure more effectively. For those working in labs or industrial plants, it arrives as a clear, colorless to pale yellow liquid, packaged in tightly sealed drums to avoid premature hydrolysis. Producers label it precisely because moisture easily reacts with it, making careful handling a must.
Vinyltriacetoxysilane’s appearance makes it fairly easy to recognize in the lab. It carries a strong, vinegar-like odor, a clue to the volatility of the acetoxy groups. The liquid weighs in at about 1.08 grams per milliliter, and its boiling point sits just above 140°C. It mixes well with many organic solvents, such as toluene and alcohols. When exposed to water, especially in humid air, the acetoxy groups hydrolyze to produce acetic acid, creating a sticky silanol intermediate. The reactivity gives it versatility, but also demands respect from handlers.
Labels typically display purity, usually over 97%, and lot-specific values for refractive index, density, and acetoxy content. Manufacturers keep close tabs on water content, advising storage in sealed containers, away from moisture and reactive chemicals. Safety guidelines underline the potential for generating acetic acid vapors, especially during usage in open systems. Proper product stewardship includes regular monitoring of batch quality and clear hazard labeling in compliance with global chemical regulations, such as GHS and REACH.
Making vinyltriacetoxysilane relies on direct reaction between vinyltrichlorosilane and acetic acid or acetic anhydride. The process forms hydrogen chloride or acetic acid as by-products, so facilities use scrubbers or neutralizers. Sometimes, organotin or quaternary ammonium catalysts speed up the reaction. The industrial scale preparation focuses on efficient removal of by-products and minimizing residual chlorides, since pure vinyltriacetoxysilane works better in downstream applications. Anhydrous conditions extend shelf-life and cut down on unwanted hydrolysis during storage.
Chemists take full advantage of the vinyl group and the hydrolyzable acetoxy arms. The vinyl end can undergo addition reactions, such as polymerization or crosslinking with unsaturated resins under radical conditions. Hydrolysis of the acetoxy groups opens the door for silanol formation, which bonds to glass, metal oxides, and other minerals through condensation. In coatings or sealant manufacturing, these twin reactivities let formulators design interfaces that stick and last longer under stress. Further modifications can replace one or more acetoxy groups with other alkoxy groups, tuning the silane for different speeds of hydrolysis or changing the compatibility with certain resins.
You might find vinyltriacetoxysilane listed under several names: triacetoxyvinylsilane, triacetoxy(ethenyl)silane, or by catalog numbers in chemical supply lists. Big producers often assign their house brands, but the CAS Number 4130-08-9 remains the anchor for accurate identification. Anyone working with regulatory documentation relies on this number for import, export, or handling, regardless of the trade name.
Handling vinyltriacetoxysilane means respecting its reactivity and volatility. Contact with moisture quickly leads to acetic acid fumes, which can sting eyes, nose, and lungs. Storage tanks or drums need vapor control and desiccants. Chemical operators wear full personal protective equipment—gloves, goggles, and sometimes respirators. Spill management plans focus on rapid containment and adequate ventilation. In larger plants, automated pumping and closed systems keep workers away from freshly opened containers. Emergency plans call for immediate neutralization of spills with sodium bicarbonate to limit acetic acid release. Data sheets advise regular training and strict documentation for storage temperatures, containers, and first aid measures. Regulatory bodies in North America, Europe, and Asia all emphasize engineering controls, exposure limits, and record-keeping in compliance audits.
Vinyltriacetoxysilane finds its way into industries building tomorrow’s cities and digital infrastructure. In construction, it anchors adhesives that glue glass, metals, or stones to modern facades. Polymeric sealants mixed with this silane last longer when battered by sun and rain. Electronics manufacturers lean on it to create durable insulating layers, improving device performance and reliability in tough conditions. My own experience with composites taught me to check for silane treatment when hunting for products that don’t crack or delaminate after a few heat cycles. In automotive factories, the silane upgrades paint adhesion on aluminum and steel, extending the life of lacquer coats. You’ll find it in wires, cables, laminates, and even specialty contact lenses, each harnessing the silane’s blend of flexibility and chemical resilience.
Laboratories worldwide keep experimenting with new ways to harness the reactivity of vinyltriacetoxysilane. Teams in Asia and Europe have tried blending it with nano-additives, like graphene or carbon nanotubes, to produce coatings that repel water or resist corrosion far beyond what older formulations offered. Others focus on green chemistry, searching for less hazardous by-product generators during hydrolysis or aiming for bio-based acetoxy groups. Polymer chemists chase after new copolymerization techniques, embedding the silane into novel resins for 3D printing or high-performance aerospace composites. Every advance carries lessons about how minor tweaks to the silane’s side groups can amplify or suppress its role in crosslinking networks, which means chemists keep a close eye on analytical testing and real-world field performance.
Acute toxicity testing tells us vinyltriacetoxysilane doesn’t rank among the worst hazards, but lab animals still develop mild irritation or respiratory discomfort during exposure, mostly because of the quick formation of acetic acid. Chronic exposure data remain scarce; toxicity studies stress the need for smart handling and solid engineering controls. Most long-term assessments track workers for skin, eye, or lung sensitization, with the main risks traced to repeated inhalation of vapors or unprotected splashes. Waste management teams push for complete hydrolysis and neutralization before sending residues for disposal, minimizing groundwater contamination risk. As more factories push for green certification, health and safety audits increasingly review silane processing histories and medical surveillance records for exposed staff.
Looking ahead, vinyltriacetoxysilane seems poised to play a bigger role as engineers and designers demand stronger, lighter, and more durable materials. Growth in renewable energy—think wind turbines and solar panels—has driven up the need for silane-modified composites that can withstand wild temperature swings and constant UV exposure. As infrastructure ages across developed nations, engineers call for sealants and adhesives that don’t give out after a couple of decades; that kind of longevity depends on high-performance coupling agents. In digital manufacturing, 3D-printed electronics and wearable devices push the limits of resin and interface design, which leads back to the versatility of organosilicon chemistry. Regulatory shifts continue to challenge producers, so future work hinges on greener processes and improved worker safety, all while keeping performance at the cutting edge. Vinyltriacetoxysilane won’t solve every problem in material science, but its track record shows plenty of reasons for chemists and engineers to keep making room for it in tomorrow’s toolbox.
In the world of construction and manufacturing, the stuff nobody talks about often holds everything together. Vinyltriacetoxysilane offers just that: a way to connect silicone and other materials in everyday products. Take a look at construction sealants or adhesives—the reason they stay put is often thanks to this very compound. When it reacts with moisture, it starts a process called crosslinking that hardens the silicone and locks everything together. This reaction is what helps prevent leaks at window edges, stops drafts at doors, and keeps insulation where it belongs. Polyvinylchloride (PVC) pipes, too, owe some of their watertight strength to how this silane works inside their walls.
Skimping on the right silane makes a difference in how long seals last. Cheap materials might hold at first, but over time, sunlight, water, and changes in temperature can eat away at the bonds. Once those bonds break, mold finds a home and water damage creeps in, turning a minor repair into a major headache. Vinyltriacetoxysilane brings a reliable option to the table. It’s been widely tested, and the safety data sheets back up its good record in industrial settings. People in the trades stick with it because projects using the right silane usually mean fewer callbacks down the road.
Using effective chemicals means less waste. Fewer repairs, less ripped-out caulk, fewer trashed sealants at the landfill. While no chemical comes without environmental concerns, manufacturers have found ways to keep residuals of vinyltriacetoxysilane to a minimum. Workers handling it need to gear up properly—protective gloves, good ventilation—because it can irritate the skin and lungs. But compared with some older choices, this compound breaks down well and causes less harm when it meets water or soil in small amounts. Keeping our buildings dry in turn keeps heating and cooling needs down, making a small dent in energy bills and carbon footprints.
I remember replacing the caulking around windows in an old family house. Before learning about which sealant works best, I kept getting gaps, mold, and peeling edges. One switch to a higher-quality silicone, built with the help of vinyltriacetoxysilane, and the problem stopped. It didn’t shrink or crack through the winter and stayed clean even as years went by. Tested formulas make a huge difference, not just the label or brand.
Companies in Europe and North America rely on this compound because of strict regulations on building safety. Inspectors check product quality, and building codes require standards that keep toxic breakdown products out of homes and groundwater. Those rules push chemical companies to keep improving their recipes for both performance and safety. Plenty of labs track the byproducts released during curing, making sure they stay within safe limits, and publish results for builders and regulators to review.
Some researchers are working to tweak the molecule for even less environmental impact or to improve how it bonds in humid or cold climates. It’s always a balance: keep the chemistry strong, make the application safer, and aim for long-term reliability. If more people push for building materials made with this sort of attention to chemistry and life cycle, houses could last longer and use fewer resources down the line. My own experience fixing things has taught me that small changes in inputs—down to single molecules—can mean a lot once you see the results, year after year, in something as simple as a dry, draft-free room.
Vinyltriacetoxysilane isn’t the sort of compound you want splashing around without a plan. This clear, nearly colorless liquid has a sharp, acetic odor that tells you right away: keep it away from your skin, eyes, and airways. Once, I uncapped a bottle in a cramped lab and caught a noseful—my eyes watered and my sinuses felt fried. The scent scratches and hangs around, so good ventilation makes a world of difference.
Acetic acid releases easily from this chemical, especially if it interacts with moisture. It reacts fast with water and even humid air, kicking off that familiar vinegar sting. Touch or inhale too much, you’ll end up with burns or a rough coughing fit. One slip might leave you with a chemical rash. My biggest lesson? Never trust a leaky seal or skip gloves, even for a quick check.
The bottle needs a spot that’s cool and bone-dry. Direct sunlight speeds up decomposition, and warm rooms only add to the risk. Humidity’s a real problem too, not just because of spoiling the product, but due to the threat to your lungs and skin. A shelf in a temperature-controlled chemical storage room, with no pipes sweating above, gives some peace of mind. Stack it next to a sink, or in a janitor’s closet, and you’re asking for trouble.
Sealed containers matter. Even the tiniest leak invites water to creep in, breaking down the silane into acetic acid, which destroys both your product and the container itself. I learned early that metal or glass containers, tightly capped, serve the job best. Forget using any old plastic jug. Over time, some plastics get weak and risk cracking. Always mark dates on containers. Anything past expiration, toss it—degraded silane might surprise you with a pressure buildup or corroded cap.
Handling comes down to routine and discipline. Never pipette by mouth, never skip goggles, and always pull on chemical-resistant gloves before opening anything. Latex isn’t enough—nitrile or neoprene gloves keep the stuff out. Labs and warehouses ought to run safety drills. Accidents become drama without a practiced response: a friend grabbed the wrong bottle once, spilled a splash on his wrist, and forgot where the nearest wash station was. Quick reactions mean everything.
If vinyltriacetoxysilane spills, contain with absorbent material—minerals, not sawdust, which can smoke or ignite. Scrub the space clean with lots of soap and water, airing things out the whole time. I’ve found even a dollop left behind starts to etch workbenches and stink up the place for days.
We can’t ignore fire hazards. Vapors could spark with static or open flames, especially in small closed spaces. A Class B fire extinguisher belongs right next to any storage area. I always check labels before walking away—one time I nearly stored this next to a strong base, which could trigger a hazardous reaction. Segregating acids, bases, and reactive chemicals prevents mixing disasters.
Waste should never go down regular drains. Collect waste in a marked, tightly sealed container and pass it on for hazardous material disposal. Simple rule: if you’re not sure what’s in a container, treat it as hazardous.
Many accidents root in rushing or cutting corners. Safety starts with real, clear labels and stays with discipline through every step. Training people right and sticking to strict routines saves gear, keeps everyone healthy, and protects the environment. Building a culture of vigilance means nobody tries to “just get it done” when risks are real.
Vinyltriacetoxysilane pops up a lot in the world of adhesives and sealants. Manufacturers love it for its ability to help paints and glues stick to surfaces, especially plastics and glass. This chemical also plays a role as a crosslinking agent in making silicone rubbers more durable. It’s a workhorse in modern construction and manufacturing, but with anything that sounds this complex, folks want to know if it’s safe.
Breathing in, touching, or even accidentally swallowing Vinyltriacetoxysilane can set off a range of health issues. Safety data sheets lay it out plain—this stuff irritates the skin, eyes, and lungs. Anyone who’s worked with it knows the strong vinegar-like odor that hits the nose. That comes from acetic acid, which releases when Vinyltriacetoxysilane touches water or moisture in the air.
According to the European Chemicals Agency, direct contact brings on stinging or burning sensations and sometimes chemical burns if left too long. Inhaling the fumes can trigger coughing, throat irritation, or shortness of breath. Spills, even small ones on a bare hand, feel unpleasant almost right away.
So what about regular exposure? Long shifts in a poorly ventilated lab or repeated splashes cause more than just bad memories—they heighten the risk of lasting skin problems or more severe lung irritation. There’s not much good evidence that Vinyltriacetoxysilane causes cancer or major organ disease in humans, at least with average workplace exposure. That being said, rats exposed to high doses in studies didn’t fare well, developing respiratory and digestive issues.
From my own time on a factory floor, I remember the stern warnings that came with handling silane-based additives. A moment's carelessness led to headaches and red eyes, and no one needed a chart to know why the gloves and goggles mattered. Those experiences stick, even decades later.
Vinyltriacetoxysilane doesn’t hang around in the environment for long. Water and sunlight break it down pretty efficiently, and it turns into harmless byproducts. Some small aquatic life take a hit during initial breakdown, but long-term buildup isn’t a huge worry here.
Companies must keep wastewater from direct disposal and follow local rules to avoid hurting fish and streams. Responsible handling, in my book, starts with proper waste management.
Most folks working around Vinyltriacetoxysilane feel safe if employers take the simple steps—ventilating workspaces, using chemical-resistant gloves, and making protective eyewear standard. Emergency showers and eyewash stations near mixing zones aren’t just for show; people use them. Labeling and regular training drills turn good intentions into real habits.
Outside the plant, most consumers rarely come into direct contact with this chemical. It sits deep in cured caulks or finished plastics, far removed from skin and lungs.
Making any potentially dangerous workplace safer never falls on just one person. It takes everyone in the loop, from purchasing to waste disposal, to follow the rules—and to call out shortcuts before they become disasters.
Vinyltriacetoxysilane offers clear value in industry, and with strict handling, health risks go down sharply. Cleaning up spills right away, airing out work rooms, and investing in safety training shouldn’t be optional. Altogether, those steps keep the benefits high and the hazards manageable for everyone involved.
Vinyltriacetoxysilane serves as a powerful chemical for industrial bonding, coatings, and formulation work. No stranger to chemistry labs, this silane compound releases acetic acid on contact with water, creating a pungent smell and, more importantly, corrosive conditions. I once walked into a workshop where someone had spilled some and failed to ventilate the area: eyes burned and throat scratched up — a lesson on its hazardous nature that stuck with me. Overlooking safety carries serious consequences for workers and the natural world.
Pouring leftover silane down the drain might feel like a quick fix, but this shortcut means acetic acid ends up in wastewater, damaging pipes, aquatic life, and eventually mixing into drinking water. Incorrect disposal leads to chemical burns, air pollution, and poorly managed hazardous waste sites. The average person likely never sees these effects directly, yet the chain reaction is easy to imagine: unhealthy air in the neighborhood, toxic runoff near rivers, or even workplace accidents from lingering fumes. These health risks can hit home for workers, nearby residents, and animals alike.
From my own time around materials like Vinyltriacetoxysilane, the rule stays clear: never open a container unless protected with chemical-resistant gloves, old clothing, proper goggles, and solid ventilation. Spills get neutralized quickly with absorbent, non-combustible material and sealed in a rugged container. Letting it react with moisture creates a sticky, acidic mess, so keeping the chemical far from open water drains or trash chutes is common sense.
Every shop tackling this chemical ought to build relationships with certified hazardous waste disposal services. Licensed outfits take over, using chemical incinerators or secure facilities to break down silanes at high temperatures, with scrubbers catching toxic releases. Some large companies use dedicated waste tanks for collection, never mixing incompatible wastes, then schedule regular pick-ups to send everything off to be rendered harmless.
Each state and city has specific hazardous waste rules, so checking local guidelines becomes standard practice. Never mix the silane with domestic or unknown waste streams, since accidental reactions spell danger — not just to pipes, but also to cleanup workers. A few labs have set up a tagged-waste system, where every container carries a record of what’s inside, date, and who’s responsible. That accountability keeps surprises to a minimum during storage and transport.
Spending a few minutes each week reviewing chemical inventories helps avoid over-ordering, so fewer containers become waste. Many workplaces invest in staff training to make sure whoever opens the bottles also knows how to close out a waste log and file paperwork for scheduled disposal. This hands-on attention benefits everyone in the workshop. When disposal becomes part of everyday routine, mistakes drop, and the work environment becomes safer.
Responsible management of hazardous chemicals like Vinyltriacetoxysilane centers on protecting human health, safeguarding water and soil, and building trust between industries and the communities around them. It takes practice and attention, but the rewards travel far beyond the workbench.
Out on factory floors, in construction sites, and behind the scenes in electronics, vinyltriacetoxysilane plays a much bigger role than many folks realize. This silane coupling agent doesn’t fill headlines, but its work speaks volumes across industries. My background in materials science opened my eyes to just how much we rely on clever molecules like this one.
In the world of construction, tiny details add up. The durability of sealants, adhesives, and silicone-based products often depends on something you can't see: how well the pieces stick together at the molecular level. Vinyltriacetoxysilane works as a chemical bridge between organic polymers and mineral surfaces—think glass, metals, concrete. This connection matters when you want long-lasting fills around windows or a leak-proof bathroom. Builders have seen fewer call-backs and better weather resistance because those bonds stand the test of time.
Take a look inside a power cable, and you’ll notice more than copper. The insulation layer isn’t just plastic—it needs to stay flexible, resist cracking, and cling tight to the wire. Vinyltriacetoxysilane helps treat and cross-link the polymer, locking the insulation right where it should be and giving it the right level of toughness. Factories pushing out miles of cable every year know that skipping this additive means shorter cable life and headaches for utility workers.
Waterproofing isn’t just about slapping on a thick coat of something. Surfaces like stone and concrete don’t always let sealers grip. Vinyltriacetoxysilane makes surfaces more receptive by reacting with moisture and bonding to them, forming a water-repellent barrier. I remember watching demo tests, water just beading up and rolling off a treated wall. You won’t hear homeowners complain about rising damp or crumbling facades when they get these treatments right.
Beneath the shiny exterior of cars and appliances, manufacturers look for ways to make sure paint, adhesives, and coatings stick for good. Vinyltriacetoxysilane’s knack for bonding dissimilar materials helps keep body panels rust-free and glued parts from coming loose. The savings in warranty costs alone justify its presence in a production line.
Some silane chemicals draw concern over byproducts and workplace fumes. Acetic acid, released during the curing process, needs ventilation. My years in compliance underscore why training and good extraction ventilation keep folks safe. Regulations ask manufacturers to measure and reduce emissions, and the industry has started finding ways to make these processes cleaner—like reclaiming or scrubbing the acidic vapor released during application.
Companies staying up-to-date with safety data, worker education, and optimized use help prevent both product waste and workplace incidents. Sharing these lessons is vital as developing regions ramp up their use of materials like vinyltriacetoxysilane. Open communication between manufacturers, regulators, and end-users keeps industry on the right track, sparking innovation in both product quality and safety.
Vinyltriacetoxysilane might not be a household name, but it holds a crucial spot in making modern materials tougher, safer, and more reliable. Practical improvements—better bonds, longer product lifespans, reduced environmental impact—matter most on the ground. With the right balance of chemistry, care, and regulation, the benefits outweigh the risks.
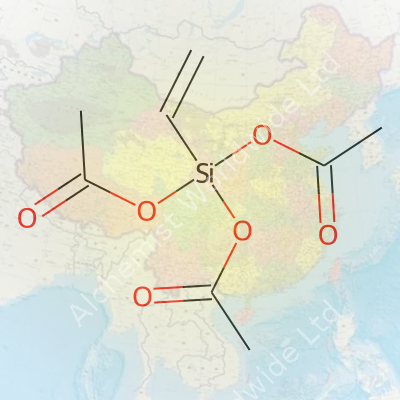
| Names | |
| Preferred IUPAC name | Triacetoxy(ethenyl)silane |
| Other names |
Triacetoxyvinylsilane Vinylsilane triacetate Triacetoxy(vinyl)silane |
| Pronunciation | /ˌvaɪ.nəlˌtraɪ.əˌsiː.tɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 2224-33-1 |
| Beilstein Reference | 1460767 |
| ChEBI | CHEBI:87154 |
| ChEMBL | CHEMBL4296841 |
| ChemSpider | 16211255 |
| DrugBank | DB16645 |
| ECHA InfoCard | 03-2119475791-44-XXXX |
| EC Number | 431-920-9 |
| Gmelin Reference | 70030 |
| KEGG | C18607 |
| MeSH | D014738 |
| PubChem CID | 13928787 |
| RTECS number | VV7310000 |
| UNII | E1P86728B5 |
| UN number | UN2920 |
| CompTox Dashboard (EPA) | DTXSID5020662 |
| Properties | |
| Chemical formula | C8H12O6Si |
| Molar mass | 248.29 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Acetic acid-like |
| Density | 1.12 g/mL at 25 °C |
| Solubility in water | Reacts with water |
| log P | -0.3 |
| Vapor pressure | 0.5 hPa (20 °C) |
| Acidity (pKa) | 10.8 |
| Basicity (pKb) | pKb: -3.4 |
| Magnetic susceptibility (χ) | -6.49e-6 cm³/mol |
| Refractive index (nD) | 1.410 |
| Viscosity | 2 mPa·s |
| Dipole moment | 3.44 D |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02,GHS05 |
| Signal word | Danger |
| Hazard statements | H226, H314, H317, H331, H335, H411 |
| Precautionary statements | P261, P264, P280, P305+P351+P338, P310, P302+P352 |
| NFPA 704 (fire diamond) | 2-1-2 |
| Flash point | 110 °C |
| Autoignition temperature | '450 °C' |
| Lethal dose or concentration | LD50 (oral, rat): 1460 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1460 mg/kg |
| NIOSH | WXQ787 |
| REL (Recommended) | 400 ppm |
| Related compounds | |
| Related compounds |
Vinyltrimethoxysilane Vinyltriethoxysilane |