
Working in chemical labs and manufacturing environments for years, I’ve watched compounds like vinyltrimethoxysilane transform the landscape of plastics and construction. Discovered during the expansion of organosilicon chemistry in the mid-20th century, the search for better coupling agents led scientists to this synthetically versatile molecule. Interest picked up steam as companies sought ways to make plastics stronger and cable insulation more resistant to moisture. Vinyltrimethoxysilane soon found its place in countless applications, from wire and cable factories to the coatings industry, fueled by research that recognized its ability to react with both inorganic and organic materials. Over decades, refinement in production methods made the compound more accessible and high in purity, pushing innovation further.
Vinyltrimethoxysilane entered the commercial stage as a clear, colorless liquid with a sharp, characteristic odor. Marketed under names like VTMO, A-171, or Silquest A-171, it soon became a staple for anyone bonding polymers to glass or minerals. Instead of acting as a simple ingredient, it bridges the worlds of plastics and inorganic fillers, turning brittle composites into durable products. As demand increased from the cable industry and construction sectors, manufacturers refined supply chains, focusing on purity and consistent volatility. Its main draw? The unique blend of a vinyl group and three methoxy substituents, creating rare flexibility for both processing and end-use.
From hands-on experience, handling vinyltrimethoxysilane isn’t like dealing with your typical monomer. With a boiling point of around 123°C and a flash point near 23°C, it evaporates fast and carries a mild risk of flammability. It weighs in at about 1.07 g/mL and, as a non-viscous liquid, flows easily across lab benches or factory flooring. Being reactive with water, this silane releases methanol on contact with moisture, quickly shifting to silanols that hook into inorganic surfaces. Its dual nature, blending a vinyl group that welcome free-radical reactions with hydrolyzable methoxy arms, gives chemists a rare degree of control.
Manufacturers establish clear specs for purity, typical levels hovering around 98% or higher. Labels must display CAS number 2768-02-7, hazard pictograms pointing to flammability and toxicity, and warnings about the formation of methanol. You’ll find drums and IBC totes stamped with batch numbers, shelf life recommendations, and storage guidelines that steer users away from humid and high-temperature environments. All these steps signal a culture of accountability, shaped by years of regulatory pressure and industry mishaps when standards slipped.
Commercial synthesis usually starts with vinyl chloride and trimethoxysilane under the catalysis of platinum complexes. Process engineers tweak temperature and pressure for maximum yield, managing the by-products to prevent unwanted fogging in the final liquid. From what I’ve seen in production facilities, even small deviations in raw material purity or reactor temperature mark a visible change in product performance. Removing impurities from the feedstock, vacuum distillation, and tight control over moisture all play a part in producing high-grade vinyltrimethoxysilane destined for medical devices or high-voltage cable jackets.
Vinyltrimethoxysilane stands out for its clear potential in polymer crosslinking. In the presence of peroxides, its vinyl group takes part in free-radical polymerization, creating robust siloxane linkages. Exposure to water or acids starts hydrolysis, releasing methanol and forming silanol groups that bind tenaciously to mineral fillers like silica and aluminum trihydrate. Chemists often modify it further—grafting onto polyolefins or formulating it into adhesives—to fine-tune mechanical and chemical resistance. With advancements in catalyst technology, its reactivity gets harnessed more efficiently, leading to products that outperform old silane-treated goods.
In my own work, tracking product identity means knowing the market’s aliases: VTMO, 3-(TriMethoxysilyl)ethylene, Silquest A-171, or Geniosil GF 56. Every brand wraps the same molecule in its labeling, though differences in purity or stabilizers can set them apart. Websites and catalogs regularly list these names for global buyers keeping tabs on REACH or TSCA listings. This variety of monikers causes confusion for new users, who soon learn that the core chemistry always circles back to a triad of methoxy arms on a vinyl backbone.
Toting flammable and toxic warnings, vinyltrimethoxysilane demands a strict routine. Splash it on skin or breathe in the vapor, and users deal with irritation, dizziness, or worse from accidental methanol exposure. Safety Data Sheets mark out air-change requirements, nitrile gloves, antistatic shoes, and flameproof cabinets. Labs and production sites install gas detectors calibrated to sense even small leaks. After several near-misses with leaking pumps, our team ramped up emergency drills and spill-response kits. Regulations like OSHA, GHS, and local fire authorities have driven everyone to harden their approach, knowing the risk rides with every drum in storage.
I’ve seen vinyltrimethoxysilane do its best work as a coupling agent in silane crosslinked polyethylene (PEX) pipes, insulation jacketing for high-tension power cables, and tough windshield adhesives. Manufacturers add it in small doses to coax plastics and fillers into chemical harmony. Its vinyl group transforms during extrusion, allowing cable and pipe makers to produce water-resistant, durable coatings that survive underground abuse and shifting temperatures. In fiberglass-reinforced plastics and paints, this silane strengthens bonding, boosts weather resistance, and extends product lifespan, which matters for wind turbines and automotive parts. Its reach stretches even to electronics encapsulation and sealants meant to survive salty sea air.
The pace of research on vinyltrimethoxysilane continues to pick up as companies target better environmental profiles and stronger products. Labs refine catalyst systems, testing hybrid formulations with silanes, titanates, or zirconates to edge out improved compatibility and lower VOC emissions. University projects run side-by-side with industry groups, pushing for alternatives that offer the same sturdy bonds with less methanol. Years of accumulated data and global patent filings prove there’s no drop in momentum, as cross-sector collaborations test novel uses in medical and aerospace markets.
Toxicologists focus on two fronts: acute exposure in factory and lab settings, and long-term environmental breakdown. Ingestion or inhalation releases methanol, risking harm to nerves or eyesight, so animal studies and cell culture experiments set human safety indices. Studies on aquatic life point to moderate toxicity, driving more careful wastewater treatment guidelines. Research highlights show most workplace incidents link back to failed personal protective equipment or improper storage, underlining the need for regular safety audits and training. Environmental scientists analyze the breakdown rate of hydrolyzed fragments, working on solutions to avoid groundwater contamination.
Vinyltrimethoxysilane sits at a crossroads. The world demands stronger, longer-lasting, and safer materials, pressing chemical producers to find lower-emission synthesis or drop-in replacements with less hazardous profiles. Trends in recyclable plastics, smarter coatings, and high-speed data cabling keep demand steady. The push for green chemistry and tighter government oversight may reshape manufacturing practices. New routes that start with bio-based feedstocks or create less hazardous by-products could take the industry in promising directions. In my view, experts and industries working together, guided by past lessons and new discoveries, will decide how far vinyltrimethoxysilane’s reach will extend in tomorrow’s world of advanced materials.
Walk down the aisles of any home improvement store, grab a tube of caulk, or open the hood of a car, and somewhere along the way, vinyltrimethoxysilane has likely played a quiet but crucial role. Chemists refer to it as a silane coupling agent. In plain language, that means it’s the bridge builder between things most folks wouldn’t expect to bond well together—like certain plastics and glass, or rubber and metal. Why does this matter? Try to picture car windshields that peel off after a few potholes, or wiring insulation that crumbles fast. The little things add up.
Let’s talk about everyday wiring. Manufacturers use this silane as a modifier in the production of crosslinked polyethylene (PEX) cables. With the silane involved, that plastic insulation wraps more snugly around copper cores. This tighter grip means wires stand up better to heat, water, and chemicals, which leads to fewer outages and safer homes. Electricians and builders trust PEX pipes and cables because they rarely fail, and vinyltrimethoxysilane helps deliver on that reliability.
This molecule’s magic doesn’t stop with wiring. It works just as well in pipes, especially for hot and cold water lines tucked behind drywall or running under floors. The PEX pipes bend easily and keep their shape, resisting cracks much better than old school PVC. That flexibility lets contractors snake piping through tight spaces. Once it’s all in place, people count on those pipes to last for decades—nobody likes mystery puddles in the ceiling. Research even shows that silane-modified PEX pipes shrug off chlorine and hot water longer, keeping repairs down and water safe from leaching.
Many adhesives, sealants, and coatings demand a molecule that acts like industrial glue—tying plastics to metals, foams to concrete, even specialty coatings to glass. Vinyltrimethoxysilane fills this role in many construction products. From laying down tough floor sealants to weatherproofing window frames, the impact is clear. I’ve seen flooring installers swelter through hot summer jobs, counting on that glue to hold tiles flat and dry for decades. If the chemistry isn’t right, repairs creep in fast, wasting money and time.
The shift to silane-based products has kept pace with growing health and safety standards. There’s less need for toxic solvents because silanes like vinyltrimethoxysilane help adhesives cure at room temperature without choking fumes. Factories and worksites get safer. Houses keep their air cleaner. These changes come from deep research—years of lab testing, government regulation, and industry troubleshooting.
Despite its strengths, some questions remain. Chemists keep searching for ways to reduce environmental impact during production and disposal. Uncured silanes and byproducts can pose hazards if mishandled. Strong ventilation and protective gear during manufacturing and use reduce risks, but new generation “greener” silanes look promising. Industry groups, researchers, and regulators weigh these innovations against cost, safety, and performance. Lower-emission, more sustainable options continue to roll out as the science advances.
From the cables behind your walls to the pipes under your feet, vinyltrimethoxysilane helps keep the modern world connected—literally and figuratively. Years of testing and hands-on building have made people trust this chemistry, but there’s always room to push for cleaner, safer ways to do the job.
Vinyltrimethoxysilane might sound like a complicated chemical, but for anyone working in construction, coatings, or adhesives, it’s a pretty common name. It serves a lot of useful purposes—acting as a coupling agent, improving adhesion, and offering resistance to moisture. Still, it doesn’t make sense to focus on what it does if we’re not also focusing on how to keep people safe around it.
My time on a factory floor has left me with a healthy respect for chemicals. With vinyltrimethoxysilane, the main concern comes from its flammable vapors and its ability to irritate skin, eyes, and lungs. I’ve seen what happens when someone ignores safety data—burnt skin, coughing fits, trips to the emergency wash station. Many workers treat it like a mild threat, but that’s a mistake you only make once.
No one walks into work hoping for a chemical burn or a breathing issue. Gloves made of nitrile or butyl rubber work well to block out the liquid. Protective goggles with side shields keep splashes out of the eyes. Folks sometimes skip the face shield, but a pair of safety glasses doesn’t always cut it against volatile vapors. Long sleeves and well-sealed lab coats or coveralls do more than just keep your clothes clean—the fabric acts as the first line of defense against skin contact.
The first time I watched an old ventilation fan sputter to a stop in a narrow workshop, I understood how fast fumes can fill a room. Fume hoods, local exhaust systems, and even simple air movers make a massive difference. Never trust that cracking a window will get the job done. Flammable vapors build up fast, especially in warm or poorly ventilated workshops. Systems with proper ducting and regular checks ensure staff aren’t working in a chemical soup.
A lot of fires started from a stack of poorly capped containers sitting next to heat sources. Storing vinyltrimethoxysilane in clearly labeled, tightly sealed cans in a cool, dry, well-ventilated spot is a no-brainer. Fire extinguishers and spill kits—complete with absorbent material and neutralizers—shouldn’t sit gathering dust. All workers must know where these items are kept and how to use them. Too many folks only figure out the spill plan once the liquid hits the floor.
Rules on a poster mean nothing unless people buy in. From my experience, regular hands-on safety sessions change how workers approach chemicals. Quick, specific drills—rinsing eyes, containing spills, evacuating—turn procedures into muscle memory. Training helps people spot problems and take action before someone gets hurt. A shared respect for what these substances can do keeps everyone a bit more aware, a bit more careful.
Companies can supply all the right gear, but without a culture of accountability, accidents keep happening. Open communication lines, routine safety audits, and easy-to-find Material Safety Data Sheets reinforce safe habits. Regular feedback loops—asking workers what’s working and what’s not—bring new hazards to light and help tweak routines.
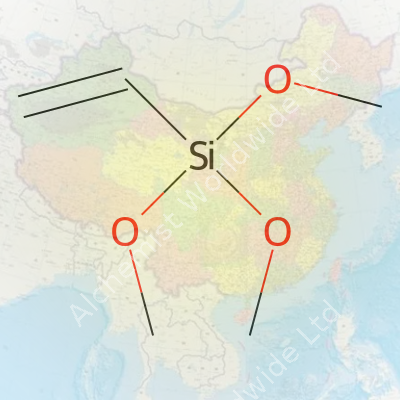
Most people outside a chemistry lab probably never cross paths with Vinyltrimethoxysilane. For those working with plastics, cables, or coatings, this compound isn’t so unfamiliar. Vinyltrimethoxysilane carries the chemical formula C5H12O3Si, a string of letters and numbers that captures its structure: five carbons, twelve hydrogens, three oxygens, and one silicon atom. Its molecular weight comes to 148.23 g/mol, which might seem like trivia until someone needs precision in a manufacturing process.
The importance of chemical formula and molecular weight shows up at the production line. Companies trust this data to ensure the right amount of silane lands in every batch of cables or pipes. A mix-up in molecular weight doesn’t just change paperwork; it can create materials that lose their strength or break down too soon. From my experience working alongside process engineers, accuracy in measurements spells the difference between a cable that lasts decades underground and one that fails months after installation.
Vinyltrimethoxysilane isn’t some random ingredient—it’s valued for helping things stick together. Acting as a coupling agent, it bonds organic polymers with inorganic surfaces. Think about the insulation around electrical wires or water pipes. The silane lets the plastic grip onto glass fibers or metals, raising durability. Without the right chemical formula, these reactions don't happen as intended, and products can start to delaminate or lose their protective qualities.
Inaccurate chemical data creates more than just inconvenience; it brings real safety worries. Anyone who’s had to recall a batch of faulty parts knows the cost isn’t just financial—reputation takes a hit too. On the technical side, an error in the molecular weight calculation can lead to dangerous errors during production, overexposure to vapors, or hazardous waste. Regulatory watchdogs require precise documentation to prevent these risks, as health and environmental safety ride on the back of getting the chemistry right.
Having witnessed regulatory audits firsthand, transparency around chemical data becomes a foundation for trust. Producers who openly share specifications and provide certificates of analysis help buyers breathe easier. There’s no substitute for traceable, reliable data. Mistakes get caught early, and end users rest easier knowing products are built to last. For Vinyltrimethoxysilane, this means sticking with reputable suppliers and keeping documentation up to the standards set by groups like OSHA and REACH.
Making sure the formula and molecular weight stay accurate calls for discipline across the board. Teams behind the scenes should double-check certificates, run routine quality control, and invest in staff training to spot inconsistencies. Digital tracking systems remove much of the guesswork and help flag mistakes quickly. In recent years, more companies have started partnering with independent labs to run spot checks. These steps help keep the industry honest, buyers informed, and products reliable.
It’s easy to overlook compounds like Vinyltrimethoxysilane unless production stops or products fail. At the heart of quality, safety, and performance lies the need to know what’s in every bottle and barrel. Reliable chemical formulas and molecular weights keep everything moving—safely and for the long run.
Vinyltrimethoxysilane isn’t just another chemical on the shelf. Anyone who’s handled it knows its sharp odor, clear liquid form, and the tricky way it interacts with moisture in the air. Some folks call it a silane coupling agent, but in a workroom, it means dealing with something that can flinch and fizz if left uncapped or left next to a drafty window. So, sound storage isn’t a checklist item—it’s insurance.
Vinyltrimethoxysilane breaks down under the wrong conditions. Left open, humidity changes it fast. Water vapor from the air hydrolyzes those methoxy groups, turning them into methanol and silanol—two things you never want escaping without a plan. Methanol builds pressure, causes drums to bulge, and no one wants a leaky, swollen container oozing hazardous material onto their shop floor.
I’ve seen old barrels stored outside, slow leaks trickling toward the storm drain. That’s trouble—legal and personal. Think back to OSHA’s views: chemicals that react with water need protection from rain, steam, or ambient moisture. A solid storage room needs steady temperatures (preferably below 25°C), absence of sunlight, and a dry atmosphere. Store those drums upright, tight-sealed, in a well-marked cabinet built for flammables. Plastic, lined steel, or original containers work fine as long as the seal holds.
A faded label leads to mix-ups, sometimes with disastrous results. Methanol vapors from degraded containers can make workers dizzy, cause fires, or simply ruin a batch of resin. Every container deserves a clear hazard sign and a date stamp. In my own workbench days, I’ve forgotten to relabel after refilling, and one near-miss was lesson enough. If you rotate stock by oldest batch, the habit keeps surprises at bay.
Fire risk follows this material everywhere. The vapor burns with a nearly invisible flame and can travel far from an open lid before igniting. Any electrical outlet or tool spark nearby turns ordinary handling into a scramble. Keep Class B extinguishers near—water won’t cut it here. Gloves and goggles hang next to the work station, not in the next room.
Routine checks save more time than they take. Walk through the chemical storage once a week. Look for swelling, rust, or sticky residues under the containers. A quick inventory lets you spot leakage early, and teaches everyone who works in the room not to cut corners. Training builds familiarity, which lowers accident rates. Centers that invest in basic chemical safety see fewer emergencies.
No one expects to witness a chemical incident—the surprise is how fast it happens. Tight controls, habitually checked, offer peace of mind. Companies committed to worker safety and regulatory compliance never regret the time they put into storage routines. For shops or labs handling vinyltrimethoxysilane, storing it like a precious tool, not a throwaway supply, pays dividends in productivity and long-term health.
Vinyltrimethoxysilane stands out in the world of industrial chemistry. Anyone who’s ever taken apart a power tool, garden hose, or even a set of windshield wipers has run across its influence, maybe without knowing it. This silane acts like a bridge, helping organic materials—a lot of them plastics—stick better to inorganic fillers or surfaces. Say you have a polyethylene cable and you want it to last for years outdoors. Add a bit of this silane, and moisture or pollutants have a much harder time doing damage. Experts keep using it because it strengthens the link between polymers and things like glass fiber or minerals. In the cable industry, this has become almost standard practice, especially in insulation for wires and fiber optics.
In construction, adhesives and sealants hold windows, flooring, and even structural panels together. Builders crave products that can shrug off water, UV light, and temperature swings. Vinyltrimethoxysilane often ends up in the formula, since it locks onto both the adhesive and the material around it. The resulting seal holds up much longer, which matters if you want your home repairs to survive the next rainy season. According to research published by the American Chemistry Council, using silane coupling agents can extend the functional lifespan of adhesive joints by years, reducing replacement costs and keeping more material out of landfills.
In the coatings industry, durability means everything. Vinyltrimethoxysilane plays a role in everything from anti-corrosive paints to coatings for concrete floors. Without a good binder, paint will flake, blister, and peel. This silane doesn’t just help the paint stick to metal or concrete; it provides a level of chemical resistance that keeps surfaces looking decent even after years of hard use. You can spot the difference in a city bridge that holds its color or a factory floor that resists staining. Based on my own work on renovation projects, applying products with this silane meant fewer callbacks to repair floors or repaint corners—a real benefit for project managers and building owners.
Automakers and consumer product designers look for ways to reduce weight while keeping products safe and tough. In glass-fiber reinforced plastics—think car bumpers, sports equipment, even electrical connectors—vinyltrimethoxysilane is one small but important ingredient. It bonds glass fibers firmly to plastic, making parts lighter but just as tough as heavier alternatives. Some manufacturers, including major European automakers, have cut the weight of structural components by up to 30% without sacrificing safety, which saves fuel and reduces emissions over a vehicle’s life.
Of course, no chemical comes without trade-offs. Vinyltrimethoxysilane releases methanol as a byproduct; care needs to be taken to avoid exposure or fire risks during processing. The industry now pushes for better ventilation, closed systems, and more robust training for workers. There’s ongoing research into alternatives with reduced hazard profiles. In my own experience consulting with small manufacturers, education and up-to-date safety protocols make a real difference in worker health and efficiency.
Vinyltrimethoxysilane shows up in products most people use daily, whether they know it or not. By linking materials that normally wouldn’t mix well, it keeps buildings standing, electronics working, and cars lighter. As industry tunes the balance between performance and safety, keeping a close eye on how and why we use this silane will shape the next wave of better, safer products.
| Names | |
| Preferred IUPAC name | (trimethoxyethenyl)silane |
| Other names |
VTMO Trimethoxy(vinyl)silane Vinyltrimethoxysilane Silane, trimethoxyvinyl- Trimethoxyvinylsilane |
| Pronunciation | /ˌvaɪ.nɪl.trɪˌmɛθ.ɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 2768-02-7 |
| 3D model (JSmol) | `3D Structure; Model: JSmol; String: C=CC[Si](OC)(OC)OC` |
| Beilstein Reference | 1461285 |
| ChEBI | CHEBI:85256 |
| ChEMBL | CHEMBL1541 |
| ChemSpider | 20479 |
| DrugBank | DB14424 |
| ECHA InfoCard | 03e8a5d9-bc7e-4198-9c18-7eaa36a5bd19 |
| EC Number | 213-934-0 |
| Gmelin Reference | 1051006 |
| KEGG | C18647 |
| MeSH | C007288 |
| PubChem CID | 62242 |
| RTECS number | YU2175000 |
| UNII | F35UQ26L9A |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID1020206 |
| Properties | |
| Chemical formula | C5H12O3Si |
| Molar mass | 148.24 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Characteristic |
| Density | 0.97 g/mL at 25 °C |
| Solubility in water | Soluble |
| log P | 0.6 |
| Vapor pressure | 1.67 hPa (20 °C) |
| Acidity (pKa) | 12.98 |
| Basicity (pKb) | pKb: 6.0 (approximate) |
| Magnetic susceptibility (χ) | -7.77×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.427 |
| Viscosity | 2 mPa·s |
| Dipole moment | 4.20 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 316.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -390.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1615 kJ/mol |
| Pharmacology | |
| ATC code | D08AX |
| Hazards | |
| GHS labelling | GHS labelling: "Danger; H226, H319, H335; P210, P261, P305+P351+P338; GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H318, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-1-1 |
| Flash point | 86 °C |
| Autoignition temperature | 335 °C |
| Explosive limits | Explosive limits: 1.1–10.5% (in air) |
| Lethal dose or concentration | LD50 (oral, rat): 7120 mg/kg |
| LD50 (median dose) | LD50 (median dose) Oral Rat: 7,130 mg/kg |
| NIOSH | VVJ1975000 |
| PEL (Permissible) | PEL: 10 ppm (TWA) |
| REL (Recommended) | 0.5-2.0% |
| Related compounds | |
| Related compounds |
Vinyltriethoxysilane Vinyltris(2-methoxyethoxy)silane Trimethoxyvinylsilane Methacryloxypropyltrimethoxysilane |